20.5: Summary
- Page ID
- 7911
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The main topics of this chapter can be summarized as follows:
| Section | Summary |
|---|---|
| 20.1 Metal Deposits | Geological resources are critical to our way of life and important to the Canadian economy. Gold, coal, iron, copper, nickel, and potash are Canada’s most valuable mined commodities. The concentrations of metals in mineral deposits are typically several thousand times higher than those in average rocks, and such concentrations only form through specific geological processes. Some deposits form within a magma chamber, others during volcanism or adjacent to a pluton, and some are related to sedimentary processes. Mining involves both surface and underground methods, but in either case, rock is brought to surface that can react with water and oxygen to produce acid rock drainage and metal contamination. |
| 20.2 Industrial Materials | Non-metallic materials are very important to infrastructure and agriculture. Some of the major industrial minerals include sand and gravel, limestone for cement and agriculture, salt for a range of applications, potash fertilizer, and decorative stone. |
| 20.3 Fossil Fuels | The main fossil fuels are coal, oil, and gas. Coal forms on land in wet environments where organic matter can remain submerged and isolated from oxygen for millennia before it is buried by more sediments. The depth of that burial influences the grade of coal produced. Oil and gas originate from organisms living in marine environments, and again, fairly rapid burial is required to preserve the organic matter on the sea floor. At moderate burial depth (2 km to 4 km), oil is produced, and at greater depth, gas is produced. Both oil and gas migrate toward the surface and can be trapped beneath impermeable rock layers in structural features, such as anticlines or faults. Some unconventional fossil fuel resources include oil sands, shale gas, and coal-bed methane. |
| 20.4 Diamonds | Diamonds originate in the mantle and are only brought to the surface by the very rare eruption of kimberlitic volcanoes. The relatively recent discovery of diamonds in Canada was based on the exhaustive search for diamond indicator minerals in glacial sediments. There are now six diamond mines in Canada. |
See Appendix 2 for answers to Review Questions.
- What are some of Earth’s resources that are needed to make a compact fluorescent light bulb? The image on the right of Figure A shows the contents of the ballast.
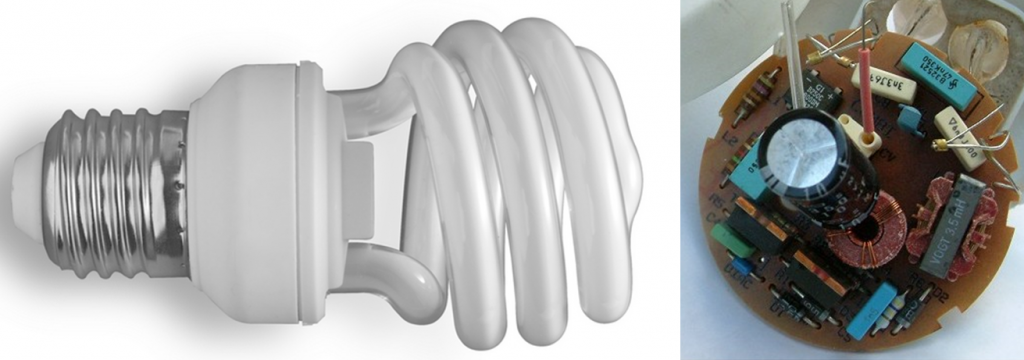
Figure A
- Explain why nickel deposits are associated only with mafic magma and not with intermediate or felsic magma?
- What is the composition of the black smoke in a black smoker, and how does that relate to a volcanogenic massive sulfide deposit?
- How might an epigenetic gold deposit be related to a porphyry deposit?
- Oxidation and reduction processes are important to both banded iron formation deposits and unconformity-type uranium deposits. Explain the role in each case.
- A typical kimberlite in northern Canada may look something like Figure B. In this case, the diameter at the surface is around 500 m, and the total depth is about 2,500 m. Bearing in mind that an open pit cannot typically be any deeper than it is wide, what mining method(s) might be most applicable to a deposit of this type?
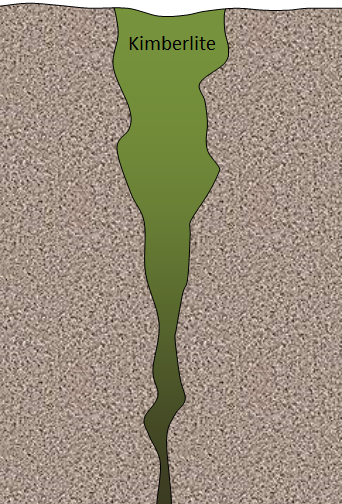
Figure B - What mineral is typically responsible for acid rock drainage around mine sites, and why is this mineral so common in this setting?
- Explain why glaciofluvial gravel is more suitable than till as a source for aggregate.
- The raw material for making cement is lime (CaO), and this is typically produced by heating limestone (mostly CaCO3) to about 1,000°C. Why is this an environmental issue?
- Name some important industrial minerals that form in an evaporite setting.
- If organic matter accumulates at an average rate of 1 mm per year, and if 10 m of organic matter is required to make 1 m of coal, how long must a swampy environment remain stable and wet in order to form a 1.5 m coal seam?
- What are the ideal characteristics of petroleum source rocks and petroleum reservoir rocks?
- How deep must the source rocks be buried to produce oil?
- Why is shale gas an unconventional reserve, and how is it recovered? What are some of the environmental issues associated with that process?
- If you were sampling glacial deposits to search for kimberlites, why would you be advised to look for kimberlite indicator minerals rather than diamonds?
Media Attributions
- Figure A (left): “Spiral CFL Bulb 2010-03-08 (white black)” © Sun Ladder. CC BY-SA.
- Figure A (right): “Elektronstarterp” © Anton. CC BY-SA.
- Figure B: © Steven Earle. CC BY.


