13.2: Ionic Crystals
- Page ID
- 17561
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)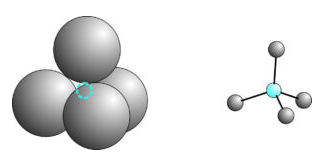
Ionic crystals are those composed of cations and anions held together primarily by ionic bonds. They have overall electrical neutrality, or else electrical current would flow until they obtained charge balance; so the total number of electrons in the structure is equal to the total number of protons. Anions repel anions, cations repel cations, so ions of similar charge stay as far apart from each other as possible. Consequently, an organized and repetitive atomic arrangement, with cations packed around anions and anions packed around cations, typifies ionic crystals. Figure 13.4, for example, shows two views of a cation (blue) surrounded by four anions. The drawing on the left does a better job of showing the relative sizes of the cation and anions but the one on the right is more clear about bond angles and distances.

Consider the mineral halite, NaCl, which contains an equal number of Na and Cl atoms (Figure 13.5a). Mineralogists have determined its atomic arrangement through X-ray studies, finding that Na+ and Cl– ions pack around each other in an alternating three-dimensional structure. As shown in the enlarged views, each Na+ bonds to six Cl– and vice versa. Bonds around one ion are all equal length and at 90° to each other. Unit cells are therefore cubic, containing four Na+ and four Cl– ions in a face-centered arrangement. Halite salt crystals, including the ones that come out of your salt shaker, are often perfect cubes.
Besides halite (NaCl), other alkalis combine with chlorine to produce alkali chlorides. Figure 13.5b, for example, shows the atomic arrangement in CsCl. The arrangement, like halite’s, is cubic. But in contrast with the halite structure, eight anions surround the alkali cation (Cs+). The difference is because Cs+ is larger than Na+. It requires more room in the crystal structure. Because there are equal numbers of Cs+ and Cl– in CsCl, if eight anions surround every Cs+, eight cations must surround every Cl–, as shown in the enlarged views.
The ionic bonds between alkalis and Cl– are not terribly strong. They break easily when salts dissolve in water, releasing free alkalis and Cl– ions. High solubility in water is characteristic of highly ionic crystals, especially those in which the ions only have charges of ±1. If concentrations of dissolved Na+ and Cl– reach high enough levels, perhaps due to evaporation, halite may precipitate from solution.

Other minerals have different atoms but have atomic structures and bonding similar to halite’s; sylvite (KCl) and periclase (MgO) are both examples. In periclase (shown in Figure 13.6), however, the ions are divalent, having a charge of ±2 (in contrast with the ions in halite and sylvite which are monovalent), and the bonds are 25% covalent. The stronger, more covalent bonds mean that periclase is harder and has lower solubility than sylvite and halite.
In some minerals, tightly bonded molecular ions, for example carbonate (CO3)2-, sulfate (SO4)2-, or phosphate (PO4)3- are present instead of simple anions. These molecules alternate with cations just as O2- and Mg2+ alternate in periclase. The molecular ions may not dissociate, even if a mineral dissolves in water, because covalent bonds hold them together. Calcite, CaCO3, is a good example. In the calcite structure, carbonate groups and Ca2+ ions alternate in three dimensions. However, the carbonate units are triangular, so the overall symmetry is not cubic like halite’s. When calcite dissolves, the ionic bonds between calcium and carbonate break easily, but the carbonate group itself does not dissociate into C and O. Consequently, dissolved species are Ca2+ and (CO3)2-, and sometimes (HCO3)–.


