13.1: The Impact of X-ray Crystallography
- Page ID
- 17560
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)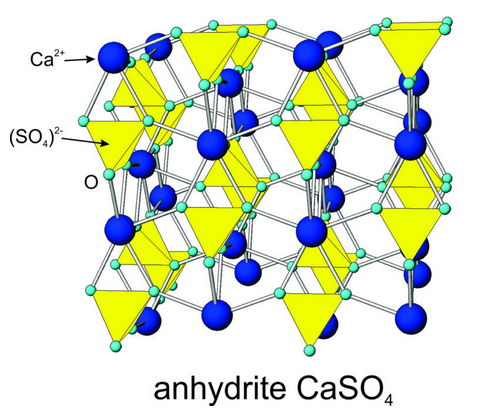
We cannot overstate the importance of the discover of X-rays and the subsequent studies by Röntgen, von Laue, and the Braggs. Before their pioneering work, scientists could not test competing hypotheses for the nature of crystal structures. Within a few decades after the discovery and development of X-ray diffraction techniques, most of the basic principles of crystal structures were well known. Figure 13.2 shows the arrangement of atoms in anhydrite (CaSO4). This arrangement was first described by J.A. Wasastjerna in 1925, based on X-ray studies. Blue spheres are Ca+2 ions and yellow tetrahedra are sulfate (SO4)2- groups. This model has passed the test of time and is accepted as the atomic arrangement in anhydrite today.
While crystallographers were working on crystals, chemists were developing atomic theory. The Bohr model of the atom, the Schrödinger wave equation, and theories of ionic and covalent bonding were firmly established by the 1920s. And things came together. Crystallographers used the new knowledge of bonding to analyze minerals. At the same time, Linus Pauling (see Box 13-1) and other chemists realized the importance of X-ray techniques and conducted X-ray studies in efforts to further understand crystal structure and bonding. In 1939, Pauling published The Nature of the Chemical Bond; he subsequently won the Nobel Prize in Chemistry in 1954.
Who Was Linus Pauling?

Linus Carl Pauling was a prolific American chemist and, in his later years, a peace and health activist. His success as a scientist stemmed from his ability to cross traditional discipline boundaries, an uncanny ability to identify key questions, and courage to put forth new, although sometimes incorrect, ideas.
Born in Portland, Oregon, on February 28, 1901, Pauling received a B.S. in chemical engineering from Oregon State University in 1922 and a Ph.D. from California Institute of Technology (Cal Tech) in 1925. For several years he was a postdoctoral fellow in Europe, where he worked with such renowned scientists as Niels Bohr, Erwin Schrödinger, and Sir William Henry Bragg. He returned to the United States and began his career as a professor of chemistry at Cal Tech in 1927.
In 1963 he left Cal Tech to join the Center for the Study of Democratic Institutions Santa Barbara, California, where he spent his time working for world peace. In the late 1960s he worked for a brief time at the University of California – Santa Barbara before moving to Stanford University. He died in Big Sur, California, on August 29, 1994, at the age of 93.
Pauling’s chemical studies covered many fields, including both organic and inorganic chemistry. One of the first to interpret crystal structures using quantum mechanics, he was also a pioneer of X-ray diffraction. His studies of chemical bonding resulted in the publication of his book The Nature of the Chemical Bond in 1939.
In the 1930s and 1940s, Pauling turned his attention to molecular chemistry, producing significant papers concerning blood, proteins, and sickle cell anemia. In 1954 he received the Nobel Prize in Chemistry “for research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances.” During his later years, Pauling received acclaim for his investigations of vitamin C. His vitamin C studies resulted in many publications including the books Cancer and Vitamin C and Vitamin C and the Common Cold, published in the 1970s.
With the development of nuclear weapons, Pauling became concerned about potential world destruction. In 1958 he published No More War!, and during the same year he delivered a petition urging the end of nuclear testing signed by more than 11,000 scientists to the United Nations. In 1962 he received the Nobel Peace Prize, making him one of only a few individuals ever to win two Nobel prizes. During his long career he received many other honors, including the Mineralogical Society of America’s Roebling Medal in 1967.


