12.1.9: What are X-rays?
- Page ID
- 18374
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)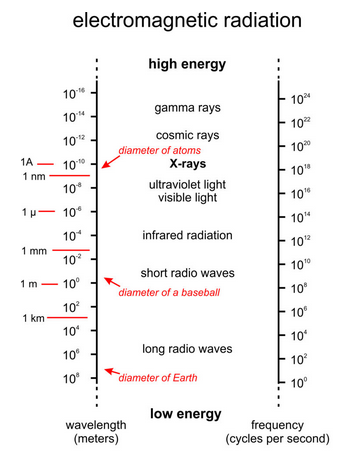
X-rays are a form of electromagnetic radiation (Figure 12.5). Whereas the wavelengths of visible light are 10-7 to 10-6 meters, X-ray wavelengths are only 10-11 to 10-8 meters. Long-wavelength X-rays grade into ultraviolet light; shorter wavelengths grade into cosmic and gamma rays. Mineralogists usually give X-ray wavelengths in angstroms (1Å equals 10-10 meters). The copper radiation commonly used in X-ray studies has λ = 1.5418Å.
The frequency (v) and wavelength (λ) of electromagnetic radiation are inversely related. Planck’s law relates them to energy:
E = hυ = hc/λ
In this equation h is Planck’s constant and c is the speed of light in a vacuum. Because of their short wavelengths and high frequencies, X-rays have high energy compared with visible light and most other forms of electromagnetic radiation. High energy allows X-rays to penetrate many natural materials, as observed by Röntgen in 1895.
X-rays of highest energy, called hard radiation, are used in many manufacturing and industrial applications, such as checking steel for flaws. X-rays of relatively low energy, called soft radiation, are used by mineralogists and for medical diagnoses. Soft radiation is the most dangerous to people because, rather than passing through tissue like many hard X-rays and gamma rays, soft X-rays interact with atoms in cells and tissues, causing damage. Because of the potential health hazards, crystallographers take special care to avoid exposure to the X-rays in their experiments.
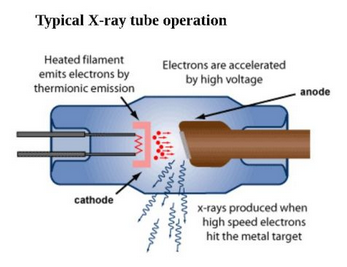
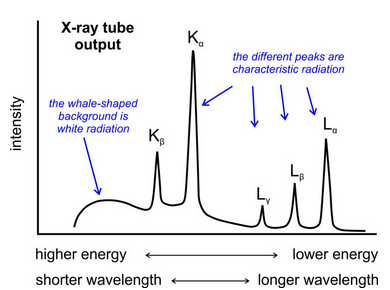
Mineralogists use X-ray tubes (Figure 12.6) to generate X-rays for diffraction studies. In the tubes, a heated filament releases a beam of high-velocity electrons that strike a metal target. Some of the high-velocity electrons that hit the target cause emission of a continuous spectrum of X-rays called continuous radiation or white radiation (the “whale-shaped” hump in Figure 12.7).
Other electrons collide with electrons orbiting atomic nuclei in the target material and bump the electrons temporarily into a high energy level. As the target electrons return to lower energy levels, they emit energy as X-rays. The energy difference between the two levels is proportional to the energy and frequency, and inversely proportional to the wavelength, of X-rays emitted. Because some electrons are elevated to higher levels than others, and because they do not all return to the same levels, typical X-ray tubes emit characteristic radiation (the peaks in Figure 12.7) having several different wavelengths. The wavelengths of the characteristic radiation depend on the metal in the target of the X-ray tube. So, X-ray tubes emit polychromatic radiation, radiation having a range of wavelengths, but most of the energy is channeled at specific wavelengths. We designate the different characteristic X-ray wavelengths using combinations of English (K, L, M) and Greek (a, β, γ) letters. The most intense peak is designated Kα (Figure 12.7).
The most common X-ray tubes have a copper target. Copper has more than one characteristic X-ray wavelength, but interpretation of diffraction results is easiest if we use one (monochromatic radiation). So we use copper’s most intense radiation, Kα, for most routine X-ray studies. To isolate Kα radiation from the other wavelengths, X-ray machines have filters, monochromators, or solid-state monochromatic detectors. X-ray tubes emit two nearly equal wavelengths of Cu Kα radiation: the wavelength of Kα1 is 1.5401Å and the wavelength of Kα2 is 1.5443Å. They are so similar that, although two wavelengths are present, for most applications the radiation is effectively monochromatic, and we take a weighted average of Kα1 and Kα2. We assume a λ value of 1.5418Å.


