9.3.2: Hydrothermal Ore Deposits
- Page ID
- 18593
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)As a melt cools and crystallizes, hot, water-rich fluids may be released. These hydrothermal fluids are rich in sulfur, sodium, potassium, copper, tin, tungsten, and other elements with relatively high solubilities. Hydrothermal fluids dissolve other elements as they flow through rocks and eventually cool to deposit minerals in hydrothermal deposits. These deposits fall into four or five categories: porphyry deposits, skarn deposits, volcanogenic massive sulfide deposits, sedimentary exhalitive deposits, and epigenetic deposits. We discuss the different types in sections below – they have distinctly different origins and vary in size from huge networks of veins covering many square kilometers to small veinlets only centimeters wide. Hydrothermal deposits generally form at mid-ocean ridges, in subduction zone, or next to plutons. In all these settings, there is a source of heat that drives fluid circulation. The exceptions are epigenetic deposits that may form in continental interiors.
The Nalunaq gold shown in Figure 9.16 is an example of hydrothermal gold. Two other examples of hydrothermal mineralization are below in Figures 9.81 and 9.82: molybdenite ore from the Keystone Mine in Colorado and gold ore from the Sierra Nevada Mountains.


Many minerals trap droplets of hydrothermal fluids that we can analyze to get information about their origins (Box 9-5). But, hydrothermal fluids may travel long distances before they deposit ore. So, determining the source of the fluid and of the ore elements is often difficult or impossible. Explaining deposition, however, is easier. It usually occurs in response to cooling, pressure changes, or changes in pH or other chemical factors.
Fluid Inclusions and Hydrothermal Ore Deposits
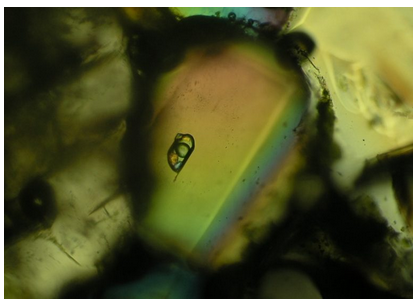
Many minerals contain fluid inclusions, small bubbles that are typically 0.1 to 1 mm in diameter, left over from the time the minerals formed. Usually we need a microscope to see them. Inclusions are found in igneous, sedimentary, and metamorphic minerals, and are of particular importance when studying hydrothermal ore deposits. Fluid inclusions are also found in deep ice cores from Greenland and the Arctic. By studying their composition climatologists gain information that helps them reconstruct the past climate record.
When trapped as inclusions, fluids are at relatively high temperatures. As the fluid cools, it contracts, and a gas bubble may form. So, many fluid inclusions contain both a liquid and a vapor bubble. If the fluid contained abundant dissolved ions, minerals such as halite, sylvite, or hematite may precipitate as well. The inclusion in Figure 9.83 contains a gas bubble, a solid crystal, and some liquid. By heating an inclusion slowly and measuring the temperatures at which any gas bubble disappears and salts dissolve, ore petrologists can often determine the conditions under which an ore deposit formed.
9.3.2.1 Porphyry Deposits
Porphyry deposits are a special kind of hydrothermal deposit. They form when hydrothermal fluids, derived from magmas at depth, carry metals toward the surface and deposit minerals to create disseminated ore deposits. These deposits are important sources of copper, molybdenum, and gold. They may also yield tungsten or tin.
In porphyry deposits, ore minerals are in small veins within a hydrothermally altered host rock, generally a porphyritic felsic to intermediate composition intrusive rock. Porphyry ores are not particularly high-grade, but the deposits are commonly large and so are profitably mined. These deposits are common in the mountains along the west coast of North and South America, and in islands of the southwest Pacific, north of Australia.
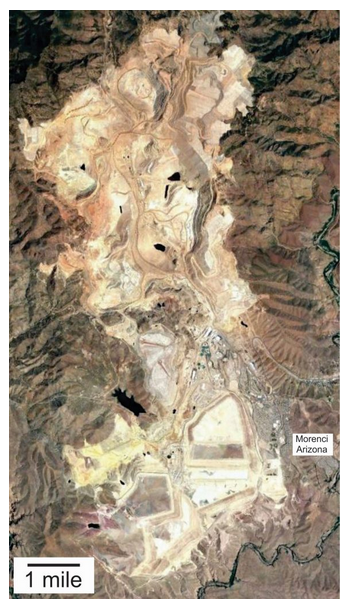
The Morenci deposit in Arizona is a porphyry deposit. Figure 9.84 shows a satellite image of the mine. The town of Morenci (pop 1,489) is in the lower right of the photo. Copper minerals were first discovered at Morenci by an Army battalion in 1865; mining began in 1872. Today, the mine, with pits that total almost 130 square kilometers, is the largest copper producer in North America. The workings extend beneath and between several large mountains next to the Morenci town site. Pyrite and chalcopyrite, both sulfide minerals, are the primary copper ore minerals, but chrysocolla (copper oxide/hydroxide) and malachite (copper carbonate) are found and mined from oxidized ore zones. Although copper minerals are by far the most important ore minerals, the mine also produces lesser amounts of sphalerite (zinc ore), galena (lead ore), and molybdenite (molybdenum ore).
9.3.2.2 Skarn Deposits

As described in Chapter 8, skarns are contact metamorphic zones that develop around an intrusion. They may be thin or thick, and their formation often involves metasomatism. Skarns can form in any kind of rock, but most are associated with limestone or dolostone. Common skarn minerals include calcite and dolomite, and many Ca-, Mg-, and Ca-Mg-silicates. Some skarns, however, are valuable mineral deposits containing copper, tungsten, iron, tin, molybdenum, zinc, lead, and gold. Skarns account for nearly three quarters of the world’s tungsten production. Less commonly, skarns produce manganese, nickel, uranium, silver, boron, fluorine, and rare-earth elements. Porphyry deposits and skarn deposits are both the results of hydrothermal activity, and a continuum exists between the two types. The photo in Figure 9.85 shows underground mining in a major skarn that yields tungsten, Canada’s Cantung Mine in the Northwest Territories.
9.3.2.3 Volcanogenic Massive Sulfides and other Exhalitive Deposits

When hydrothermal fluids create ore deposits at, or near, Earth’s surface, we call the deposits exhalitives. The Kidd Mine, shown in Figure 9.86, is an example. The mine is in eastern Ontario, near Timmins; it is the world’s deepest base metal (a term referring to industrial metals excluding iron and precious metals) mine and extends to a depth of more than 3,350 meters. The bottom of the mine is said to be the closest a person can get to the center of Earth.
The Kidd Mine started producing in 1966. It was initially an open pit but soon went underground. The moneymaking metals are mostly copper and zinc, but silver, gold, lead, and other metals are important too. Ore minerals – mostly pyrite, pyrrhotite, chalcopyrite, sphalerite, and galena – were deposited when warm, metal-rich hydrothermal waters combined with ocean waters in sediments and rocks of the ocean floor. The resulting deposits are in pods or sheets within sedimentary rock layers that, in places, contain nearly 100% ore.
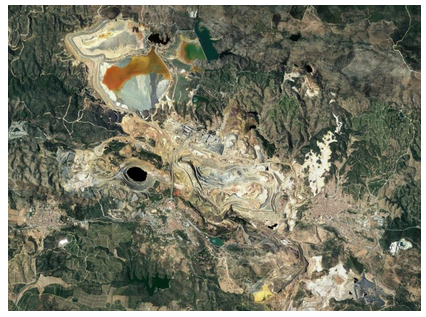
The Kidd ore deposit is an example of a volcanogenic massive sulfide (VMS) deposit. Most such deposits are small, and, although the Kidd Mine is large, it is not the largest. The largest VMS deposits, about twice the size of the Kidd, are the Windy Craggy deposit in British Columbia, discovered in 1958, and the Rio Tinto deposit in Spain, discovered in 1972. The quality of the ore in massive sulfide deposits is high, host rocks are generally greater than 60% ore minerals, so even if they are small, massive sulfide deposits are alluring mining prospects. The photo below in Figure 9.87 shows one of the main pits at the Rio Tinto Mine. The name of the mine translates to Red River, and the photo in Figure 9.88 shows the acid mine drainage that gives the river its color today. Runoff from the mine has caused major environmental problems.

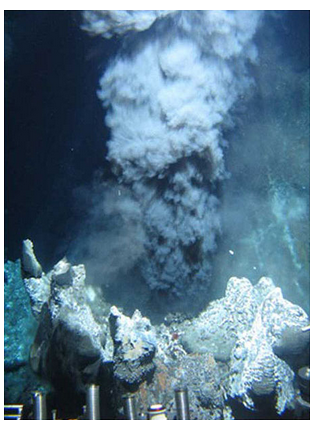
What makes VMS deposits especially intriguing is that we can see them being created today. This photo (Figure 9.89), and the spectacular YouTube video that is linked below it, show a black smoker on the ocean floor. At these smokers, hot hydrothermal waters, mixing with ocean waters, create fine particles of sulfide minerals and produce massive ore deposits. The iron sulfides that are the most common minerals created, are black, so the name. The ores mined from the Kidd, Windy, Craggy, Rio Tinto, and other massive sulfide deposits owe their origins to black smokers such as the one seen here. The smokers cover huge regions of the ocean floor and did so in the past. After forming, they later became uplifted and incorporated into the continents where we find them today. As seen in the video, black smokers are also sites of abundant marine life.
Black smokers occur at all mid-ocean ridges and mid-ocean ridge ores are potentially minable. Prospecting of ocean floors is occurring today, and some mining companies have developed tentative plans for mining operations. To date, however, the water depth has proven too great for direct mining. Some areas near black smokers contain sulfide ooze that might, perhaps, be picked up with a vacuum. The Papua New Guinea (PNG) government invested more than $100 million in the Solwara 1 project, a planned mining operation that was to target mineral-rich hydrothermal vents on the ocean floor just north of PNG. The project had significant funding problems and met with much local and environmental opposition. It was cancelled in 2019.

Sedimentary exhalitive (SEDEX) deposits are close cousins to VMS deposits. The difference is that host rocks in SEDEX deposits are sedimentary rocks. These deposits are rare compared with the other deposit types already discussed. They have produced significant amounts of zinc, lead, silver and sometimes copper. But, most of them are not economical to mine. Figure 9.90 shows copper ore (mostly chalcopyrite and bornite) from the Rammelsberg SEDEX deposit in Germany. At Rammelsberg, the hydrothermal ores are in shale. The Rammelsberg mine once produced silver, copper, and lead but is closed today.
9.3.2.4 Epigenetic Deposits
When a hydrothermal deposit is not directly associated with a pluton, we call it an epigenetic deposit. Often, the hydrothermal fluids have traveled so far that their original source is unknown. For example, some flat sedimentary rocks in the interior of the United States have strata of limestone that contain ore minerals. These include mineral deposits of the Southeast Missouri Lead District and related deposits in Iowa, Wisconsin, and Illinois. The deposits are especially concentrated in a curved zone called the Viburnum Trend in southeast Missouri. Similar deposits are found at Pine Point in Canada’s Northwest Territories, in northern, England, and in a handful of other places around the world. We call all these deposits Mississippi Valley type (MVT) deposits.

This photo (Figure 9.91) shows a museum specimen from an MVT ore deposit in the North Pennines of England. This sample is mostly green fluorite, but also contains silver-gray cubes of galena and white and salmon-colored calcite.
Primary ore minerals in MVT deposits are generally galena (PbS) and sphalerite (ZnS). Fluorite (CaF2) is common but has little economic value. Weathered or altered MVT ores may contain anglesite (PbSO4), cerussite (PbCO3), smithsonite (ZnCO3), hydrozincite (also a type of zinc carbonate), and secondary galena or sphalerite. Flowing groundwaters deposited both primary and secondary ore minerals long after limestone formation, but the origins of the groundwaters are unknown. According to some geologists, the metal-rich ore fluid came from oxidized clastic iron-rich rocks.


