9.3.3: Sedimentary Ore Deposits
- Page ID
- 18594
9.3.3.1 Placer Deposits
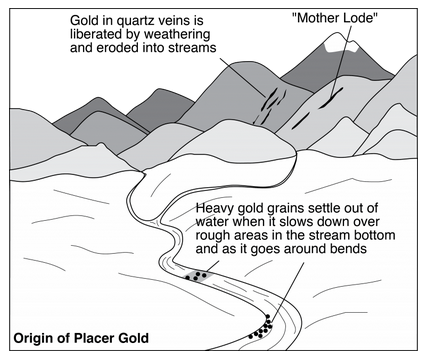
Gravity may be an important force that concentrates economic minerals. Heavy minerals, weathered from igneous, sedimentary, or metamorphic rocks, can be picked up and rivers may transport them long distances before they become concentrated in placers. So, placer deposits, also just called placers, form when one or more minerals concentrate in this way to become an ore deposit. The word placer is Spanish for alluvial sand.
Typically, placers form where a stream’s velocity slows on point bars, in braided streams, or in alluvial fans (Figure 9.92). Other similar deposits are in beach sands or gravels on ocean and lake shores. And some placers, although not commonly mined, form in offshore marine environments on continental shelves.

Placer gold set off the historically important California Gold Rush of 1849 (Figure 9.93). The original sources of minerals in placers are often difficult to determine but, in California, the source has been identified. The California gold weathered from extensive vein deposits, called the mother lode, in the Sierra Nevada Mountains. The lode is in a zone east of Sacramento and San Francisco that is 1 to 6 km wide and extends almost 200 km north-south. Mother Lode gold is in quartz veins up to 20 meters thick and thousands of meters long. Many prospectors told stories of finding and mining the mother lode, but in actuality, most of it eroded away long before the miners arrived.
The California gold rush resulted in California being admitted to the United States in 1850 and later provided a name for a San Francisco football team (the 49ers). Although once one of the most productive gold-producing districts in the United States, the Mother Lode is presently mostly a tourist destination and the home of wineries.

Placer minerals must be both dense and durable to be deposited and remain in place without decomposing. Native metals such as copper or gold, sulfide minerals such as pyrite or pyrrhotite, and oxide minerals such as magnetite or ilmenite are all dense and likely to be found in placers. Metal oxides, especially magnetite (iron oxide), are common and especially dense and durable, and often dominate such deposits. And gold is dense and extremely resistant to any kind of weathering and so can accumulate in stream and river sediments. Gold in placer deposits is found as nuggets that range from microscopic size to basketball size. The photo seen here (Figure 9.94) shows millimeter and finer-sized placer gold nuggets from an unknown origin.
Other important metals besides gold come from placers. As discussed previously, during the Bronze Age, people of Mesopotamia and Greece obtained their tin from what is today England. That tin came from tin placers in the modern-day area of Cornwall. The tin is in cassiterite (SnO2) that derived from the weathering of nearby granites. People mined the Cornwall tin deposits, placer and underground, for more than 4,100 years, beginning about 2150 BCE. The last mine, the South Crofty Mine, closed in 1998. Other important minerals found in placers include diamond, garnet, ilmenite and rutile (titanium ore), ruby, sapphire, monazite, and zircon.
9.3.3.2 Iron Ores

Sedimentary ore deposits also form by chemical precipitation; banded iron formations (BIF), found in Precambrian shields, are examples. Photos of BIF are seen here in Figure 9.95, and also in Figure 7.77 (Chapter 7). Banded iron formations are massive in scale, in places covering hundreds of square kilometers, and perhaps being tens to hundreds of meters thick. If they contain especially significant amounts of magnetite and hematite, they are profitably mined.
Typical banded iron formation contains repeating layers of black to silver iron oxide (magnetite), and red chert (microcrystalline quartz). The overall red color is because the chert contains inclusions of hematite. The outcrop shown in Figure 9.95 is in the Mesabi Iron Range of Minnesota. It formed during the Precambrian Eon, 2.1 billion years ago, and is 2 meters high, 3 meters across, and weighs 8.5 tons.
Banded iron formations include oxides, silicates, and carbonates of iron. They are most commonly rich in magnetite (Fe3O4) and hematite (Fe2O3) but siderite (FeCO3), and the iron hydroxides goethite and limonite are sometimes ore minerals. Other minerals in BIF include the carbonate ankerite (similar to siderite with impurities), and iron silicates such as minnesotaite (an iron amphibole), greenalite (Fe-rich serpentine), or grunerite (also an iron amphibole). Changes in the composition of Earth’s atmosphere more than two billion years ago caused deposition of these minerals. They are commonly associated with very old fossil algae which likely caused the atmospheric change. BIFs are found on all the world’s major continents, and, where they are found, mining often occurs.

Australia contains many large iron mines where hematite is the main ore mineral. Most large hematite deposits formed by alteration of banded iron formations. These deposits are less common than magnetite-rich banded iron formation but easier to mine and process.
Australia dominates the world iron market, producing 36% of all iron ore. Brazil and China each produce about 16%. Figure 9.96 shows an iron mine at Tom Price in Western Australia. The bedrock and the soil have the typical reddish color associated with oxidized iron minerals, such as hematite and goethite, that typifies iron formations.
9.3.3.3 Evaporites
When a body of water is trapped, evaporation can lead to precipitation of halite and other salts. Thick evaporite deposits of halite, sylvite, gypsum, and sulfur have formed in this way. Section 7.3.2 (Chapter 7) discussed the formation of these deposits and evaporite minerals. Evaporites are mined for many things, most notably halite, sylvite, and gypsum. They also produce boron- and lithium-mineral ores.
9.3.3.4 Laterite Deposits
The weathering of preexisting rock may expose and concentrate valuable minerals. Over time, water leaches rocks and soils, dissolving and carrying away soluble material. The remains, called residuum, may be rich in aluminum, nickel, iron, or other insoluble elements. In tropical climates extreme leaching has produced soils called laterites, which are rich in aluminum or, sometimes, nickel. If aluminum-rich laterite lithifies to become rock, we term it bauxite . We mine laterites and bauxites from open pits to produce nickel and aluminum and, sometimes as a secondary commodity, iron. Chapter 7 discussed the formation of laterites and bauxites. See especially Box 7.1.
9.3.3.5 Phosphorites

Phosphorus, like potassium, is an essential nutrient for all living things and people have used it as a fertilizer for centuries. In the 18th century, people used bone ash as a source of phosphorus, created by roasting the bones of animals to liberate contained phosphorus. In the 19th century, people turned to guano, or bird and bat excrement, as the principal source of phosphorus for fertilizer. They obtained guano from small ocean islands with large bird populations. Figure 9.97 shows a typical island with white guano deposits. Similar deposits are found on many ocean islands around the world, where tens of thousands to millions of years of bird excrement has collected. In some places, layers of guano are up to tens of meters thick.
Today, instead of coming from bird dung, most phosphorus comes from phosphorite – which is what is being mined in the photo below in Figure 9.98 – a phosphate-rich chemical sedimentary rock that forms in several different marine environments. The most common depositional environments are in shallow, near-shore marine settings, including beaches, intertidal zones, and estuaries. The mine in this figure is in Togo, West Africa.
Figure 9.99 shows the Bou Craa phosphorite mine in the interior of Western Sahara (a disputed territory south of Morocco). A 60-mile long covered conveyor belt carries ore from the mine to a shipping port on the Atlantic Coast where it is transported for use a fertilizer around the world. Morocco and Western Sahara contain 70% of the world’s known phosphate deposits today, but China is the number one producer. Some smaller, but still significant production occurs in other countries. In the United States, we sometimes mine phosphorite in Florida, Idaho, North Carolina, and Utah.




