9.2.4: Gems and Gem Minerals
- Page ID
- 18553
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Gems are precious or semiprecious stones and related substances that we can cut or polish to use for ornamentation. There are many kinds, and they may be natural or synthetic. Most gems are natural materials; they can be either minerals or nonminerals.
The term gemstone is sometimes used to refer to gems that are minerals. The table lists the common gemstones and their most important countries of origin. Only one or two countries dominate production of many gems, including diamond. Because gems differ in appearances from common minerals, they often have names different from their mineral names. The table contains some examples.
Diamond, emerald (a variety of beryl), and ruby (a variety of corundum), have been, historically, the most valuable gemstones. Sapphire (another variety of corundum) and alexandrite (a variety of chrysoberyl) are nearly as valuable. The most expensive gem on record sold for $71.2 million during a 2017 auction in Hong Kong. It was a unique oval-shaped diamond with a vivid pink color, weighed about 60 carats, and was almost 2 cm in longest dimension.
It is not the composition of gems that makes them valuable, but rather their appearance. Most are varieties of common minerals that exhibit spectacular color, clarity, brilliance, or play-of-color. The hardest gems – for example diamond, ruby, and sapphire – are especially highly valued because they are most durable. Besides its beauty, a gem’s rarity and uniqueness are important to some people, and gems sometimes have snob appeal.
Common beryl, is opaque with a nondescript blue color. For an example, see Figure 6.89 (Chapter 6). But exceptional beryl crystals are beautiful translucent or transparent gems, including emerald (green), aquamarine (blue), morganite (pink), helidor (yellow), goshenite (clear), and several other varieties. The four photos below (9.59 through 9.62) show gemmy examples of emerald, aquamarine, heliodor, and morganite that could be cut to make colorful gemstones. Although common beryl has no value as a gem, it is sometimes mined as a beryllium ore mineral. Other photos of gemmy beryl were seen in Figures 1.12, 1.13, and 1.14 (Chapter 1).
| Mineral Gems and the Most Important Producing Countries and Regions | ||
| mineral | gem | source |
| beryl | emerald | Colombia, Brazil, Russia, Egypt, East Africa |
| beryl | aquamarine | Brazil, Afghanistan, Pakistan |
| chrysoberyl | alexandrite | Russia, Brazil |
| corundum | ruby | Cambodia, Myanmar, Afghanistan, India, Australia, Thailand, Sri Lanka, Brazil |
| corundum | sapphire | |
| diamond | diamond | Australia, South Africa, Namibia, Russia |
| jadeite | jade | Myanmar, China |
| K-feldspar | moonstone | many sources |
| olivine | peridot | Egypt, Myanmar, Australia |
| opal | opal | Australia, Hungary, Mexico |
| quartz | amethyst | Russia, Sri Lanka, India, Uruguay, Brazil |
| quartz | citrine | |
| topaz | topaz | Brazil, Sri Lanka, Russia, India |
| tourmaline | tourmaline | Namibia, Brazil, United States, Russia |
| turquoise | turquoise | United States, Egypt, Australia |
| tremolite – actinolite | nephrite (jade) | Russia, China, Taiwan, Canada |
| zircon | zircon | Sri Lanka |






Some gems, such as the opal shown in Figure 9.63, show fire, a type of play-of-color that appears as changing flashes of different colors when we view a gem from different angles. Fire is most apparent in minerals that exhibit dispersion – the ability to separate white light into different colors that pass through the mineral along slightly different paths. Diamond best exhibits this property (Figure 9.64) but other minerals, including sphene, zircon, and a green garnet called demantoid garnet sometimes also display fire. Fire is most notable in clear gemstones and may be completely masked in strongly colored stones. Fire may also be seen in amber or pearls – which we call gems although they are not minerals. Proper polishing or cutting can enhance play-of-color in gems of many sorts.
We saw other examples of minerals that exhibit play-of-color in previous chapters; all are common gemstones:
•Figures 1.2 (Chapter 1) and Figure 3.52 (Chapter 3) – opal
•Figures 3.51 (Chapter 3) – limonite
•Figures 3.53 (Chapter 3) – labradorite
•Figures 3.54 (Chapter 3) – moonstone

Gems and other minerals derive their color from many different things (see Chapter 3). Common minerals may have little value as gems, but if we can alter or enhance colors, even common quartz may become valuable. Gemologists, therefore, often treat gems and minerals, natural or synthetic, to change or enhance their color and increase their value. The vast majority of gems on the market today have had their appearances enhanced in some way.
For instance, quartz crystals from the Hot Springs area of Arkansas are irradiated to disrupt their atomic structure and give them a smoky, purple, or black color. Irradiation is also used to induce color changes in diamond and topaz. Figure 9.65 shows a remarkable blue colored topaz crystal. The strong color was produced by irradiation.
Gemologists also change a stone’s color using dyeing or heat treatment, although dyeing only affects gems such as jade and chalcedony, that are porous. Gemologists have successfully used heat treatment – which changes elemental valences or alters atomic structures – on quartz, beryl, zircon, and topaz, although the results are not always predictable.
9.2.4.1 Synthetic Gems

Today, it is routine to synthesize gems of many sorts, including diamonds, and also varieties of quartz, beryl, corundum, and garnet. Chrysoberyl, opal, rutile, spinel, topaz, and turquoise are also synthesized. We saw photos of both natural and synthetic topaz and ruby in Figures 1.16, 1.17, 1.18, and 1.19 (Chapter 1). The photos seen here in Figure 9.66 show synthetic sapphire, ruby, and emerald.

Several synthetic compounds with no natural equivalents are used as simulants for gems. Foremost among them are yttrium aluminum garnet (YAG; Y3A15O12) and cubic zirconia (CZ; ZrO2), both used as imitation (or “genuine faux” from the French for “fake”) diamonds. This photo (Figure 9.67) shows different examples of (synthetic) cubic zirconia. Today, clear CZ – two examples are seen in the photo – is the most common diamond simulant. CZ can have just about any color and so is a common simulant for other, darker-colored, gems as well.
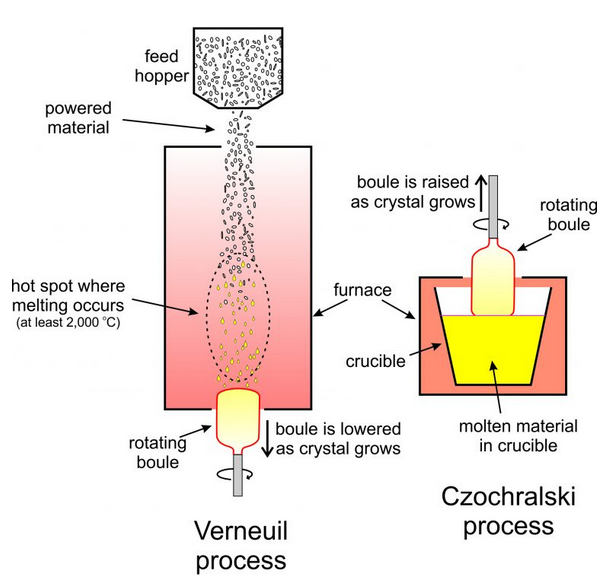
Gem manufacturers use several different methods to produce synthetic crystals. The Verneuil process and the Czochralski process both involve crystallizing gems from molten material (Figure 9.68). In the more common Verneuil process, powdered starting materials pass through a hot furnace and melt to produce droplets that add to a growing boule, a single elongated crystal, at the bottom of the furnace. The boule is slowly withdrawn from the furnace as it grows. This technique produces large synthetic rubies, sapphires, spinels, and other gems. The synthetic rubies are often key components of lasers.
In the Czochralski process (also shown in Figure 9.68), a seed crystal is placed in contact with a melt and allowed to grow. As it grows, the crystal is raised from the melt and so grows even longer. The photo on the left, below in Figure 9.69, shows synthetic corundum, including red ruby, made using the Verneuil process. The other colored stones are generically called sapphire. The stones in the bottom of the photo have been faceted to be used as ornamental gems. The photo on the right, in Figure 9.70, shows ruby being synthesized using the Czochralski process. The Czochralski process can produce 40 cm, or longer, ruby boules.

A third approach, the flux method, has sometimes yielded large synthetic crystals, notably ruby, sapphire, alexandrite, and emerald. A flux is a material that promotes reaction and crystal growth but is not incorporated in crystal structures. A mixture of chemicals – including those needed to make the desired mineral and others to act as the flux – is ground together and heated above its melting point. As the temperature is lowered, crystals begin to grow. After the melt solidifies, water or other reagents remove the flux, leaving the desired crystals. Most synthetic rubies and emeralds are created this way, but the process is very slow and may take many months. A related approach, called the hydrothermal method produces synthetic quartz and a few other gems. The method involves heating water that contains the necessary dissolved elements, and letting the crystals grow as the solution cools. It is a very slow process, like the flux method.
Mineralogists synthesize minerals in other ways, but they rarely produce gems of great value. For example, synthetic minerals may be grown from hydrothermal solutions in high-pressure reactor vessels called bombs. Synthetic quartz, corundum, and emerald have all been made this way. Synthetic diamond and a few other high-pressure minerals are made using a solid state (no melt or water) approach. This process involves a cylinder of starting material, enclosed in a graphite heater, squeezed between two pistons. Electricity passing through the graphite heats the material to temperatures at which crystals will grow. Chemists at General Electric have perfected this technique for making gem-quality synthetic diamonds.
9.2.4.2 Cutting and Polishing Gems
Most gems have shapes that do not resemble natural crystals. Sapphire, a variety of corundum, generally grows as hexagonal crystals, but when sapphires are to be incorporated into jewelry, gem cutters shape and polish them to increase their beauty and value. Figure 9.71 below shows natural sapphire from Madagascar and Figure 9.72 shows the Logan sapphire, a famous gemstone originally from Sri Lanka. Twenty diamonds surround the sapphire. Gem cutting affects value as much as the quality of the raw material. Cutting and polishing takes place in many parts of the world. Israel and Belgium dominate diamond cutting; the United States, India, Hong Kong, Thailand, and Brazil also cut significant amounts of various gems.



Gemologists shape gems in several ways. Agate, opal, chalcedony, and onyx may be tumbled in a cylinder with a polishing/abrading agent. The cylinder rotates until the stones have smooth surfaces. The stones become polished, but often have irregular shapes. Alternatively, gemologists shape stones using a wet grinding wheel made of quartz sandstone (for relatively soft minerals) or metal impregnated with diamonds (for harder minerals). After shaping and polishing, the stones are called cabochons. They have a smooth, domed top and, usually, a flat base. Until the Middle Ages, most gems were cut as cabochons. The gems shown in Figure 9.73 are all varieties of the mineral tourmaline, shaped and polished as cabochons.

While most soft gemstones receive a cabochon cut, many hard gemstones are faceted. Facets are small, polished, planar surfaces that give the stones attractive shapes and light properties. With proper cutting, originally dull stones can sparkle. Facets are usually symmetrically arranged in geometric shapes. Gemologists create them by mounting stones on a holder, called a dop, and grinding the stone with a diamond-impregnated wheel. As with cabochons, the gems are polished after being ground. Figure 9.74 shows a faceted example of orthoclase from Madagascar. Figures 9.47-9.50 (spinel), 9.64 (diamond), 9.66 (synthetic sapphire, ruby, and emerald), 9.67 (cubic zieconia), and 9.69 (synthetic ruby and sapphire) contain other photos of faceted gems.


