9.2.2: Sulfides and Sulfosalts
- Page ID
- 18534
Metallic ore deposits contain many different sulfide and related ore minerals. Most are quite rare. The table seen here lists the more important species. Pyrite (iron sulfide) is most common. Other relatively common sulfides include chalcopyrite (copper iron sulfide), molybdenite (molybdenum sulfide), sphalerite (zinc sulfide), galena (lead sulfide), and cinnabar (mercury sulfide). The others in the table are less abundant but are occasionally concentrated in particular deposits.
Sulfide minerals (such as pyrite) contain one or several metallic elements and sulfur as the only nonmetallic element. Bonding is variable: generally either covalent, metallic, or a combination of both. Metallic bonding, too, is important in some species. Other very uncommon minerals grouped with the sulfides (because of similar properties) contain selenium (the selenides), tellurium (the tellurides), or bismuth (the bismuthides) instead of sulfur. A related group of minerals, the sulfosalts, contains the semimetals arsenic and antimony in place of some metal atoms. Because many sulfides have similar atomic arrangements, solid solutions between them are common. The same holds true for the sulfosalts.
Primary sulfide minerals consist of sulfur and reduced metals. When exposed to oxygen rich-groundwaters, or to the atmosphere at Earth’s surface, they easily oxidize or break down in other ways. Oxidation can alter the original mineral’s color or texture. It can also create new minerals. So, iron-bearing sulfides may turn into iron oxide (magnetite or hematite), iron hydroxide (limonite or goethite), or iron carbonate (siderite). Galena (lead sulfide) may become cerussite (lead carbonate). Copper sulfides may become azurite or malachite (both hydrated copper carbonates).
Sulfide minerals often form in common associations. Pyrite, sphalerite, and pyrrhotite are frequently found together, as are chalcopyrite, pyrite, and bornite or pyrrhotite. In some carbonate-hosted deposits, sphalerite and galena occur together. We can depict sulfide associations using triangular composition diagrams. Box 9-4 (below) presents a detailed discussion of Cu-Fe-S ore minerals and explains how we use triangular diagrams to show solid solution compositions.
| Sulfide and Sulfosalt Ore Minerals (* = generally metallic) |
|
| sulfides | |
| *pyrite | FeS2 |
| *chalcopyrite | CuFeS2 |
| *molybdenite | MoS2 |
| sphalerite | ZnS |
| *galena | PbS |
| cinnabar | HgS |
| *acanthite | Ag2S |
| *chalcocite | Cu2S |
| *bornite | Cu5FeS4 |
| *pyrrhotite | Fe1-xS |
| *millerite | NiS |
| *pentlandite | (Fe,Ni)9S8 |
| covellite | CuS |
| realgar | AsS |
| orpiment | As2S3 |
| *stibnite | Sb2S3 |
| *marcasite | FeS2 |
| Sulfosalts | |
| *cobaltite | (Co,Fe)AsS |
| *arsenopyrite | FeAsS |
| pyrargyrite | Ag3SbS3 |
| *tetrahedrite | Cu12Sb4S13 |
| enargite | Cu3AsS4 |
Unlike other mineral groups, especially the silicates, color is sometimes a good way to identify sulfide minerals, especially for those with metallic lusters (marked with * in the table, above). The reason is that transition metals often control color, and the color of sulfides is often due to the metals they contain. So, color is helpful. Sulfide minerals, however, show lots of variation in appearance, especially if they are tarnished. Space does not permit including photos of all the different sulfides here, but some examples are below.
The most common gold and goldish sulfides are pyrite, chalcopyrite, and pyrrhotite (shown in Figures below). They can be very hard to tell apart. Pyrite, also called fool’s gold, is seen in Figure 9.31. The specimen in the photo has pyrite’s typical metallic golden color. Figures 3.2 and 3.42 (Chapter 3) show other views of golden pyrite. Chalcopyrite, in contrast with pyrite, contains copper and easily tarnishes – often to a yellow-green color. The photo of chalcopyrite below (Figure 9.33) shows multiple colors due to tarnishing. Figures 3.22 and 3.43 (Chapter 3) also show tarnished chalcopyrite. Chalcopyrite is much softer than the other two minerals which also sometimes helps identification. Pyrrhotite, seen in Figure 9.32, is the only one of the three that is magnetic, and sometimes that can distinguish it from the others. Some pyrrhotite has a more silvery color than pyrite which helps identification.



Many sulfides have a gray color, sometimes with metallic luster. The photos below show examples of galena (Figure 9.34), molybdenite (Figure 9.35), and stibnite (Figure 9.36). The lusters of the samples in these photos are not particularly metallic, but many specimens of these minerals are. For example, Figure 3.21 (Chapter 3) shows a spectacular example of metallic stibnite, and Figure 3.40 (Chapter 3) shows a hexagonal flake of metallic molybdenite.



The three photos below show copper minerals. Copper minerals are often characterized by strong colors. Bornite, which is unremarkable and hard to identify if unoxidized, commonly oxidizes and tarnishes to form what we call peacock ore (Figure 9.37). Covellite (Figure 9.38) is usually identified by its blue, commonly metallic color. We include a photo of azurite and malachite here (Figure 9.39) because of the color similarity to the other two minerals. But azurite and malachite are secondary copper carbonate hydroxides and not sulfide minerals. Figure 3.47 (Chapter 3) shows another specimen containing azurite and malachite.



Sphalerite (ZnS) is a mineral that has many different appearances; the three photos below show examples. In Figure 9.40, the sphalerite is dark colored and bordering on metallic. In Figure 9.41, two prominent calcite crystals accompany the sphalerite, which has a characteristic brown resinous appearance. In Figure 9.42, the sphalerite is almost gemmy. Gray galena and orange dolomite are also present in the photo. Because of its many different appearances, sphalerite can be hard to identify unless it is brown and resinous as in Figure 9.41, and in Figure 3.26 (Chapter 3). For example, Figures 3.45 and 3.46 (Chapter 3) showed clear green and yellow sphalerite that looks nothing like the samples seen below. The many different colors of sphalerite are due to trace amounts of iron and other elements in the zinc sulfide. In laboratories, pure manufactured zinc sulfide is white.



The photos below show three of the most colorful sulfides: cinnabar (HgS), realgar (AsS), and orpiment (As2S3). We typically identify these minerals by their color. Cinnabar (Figure 9.43) is generally a red-pink color, although the color is sometime diluted by other minerals present. Realgar (Figure 9.44) has a bright orangey-red color, and orpiment (Figure 9.45) is one of two common minerals (the other is sulfur) that is yellow. Note that the orpiment contains a small amount of orange realgar; their compositions are nearly identical. Figure 3.39 (Chapter 3) contains a photo of orpiment with calcite.



Cu-Fe Sulfide Minerals
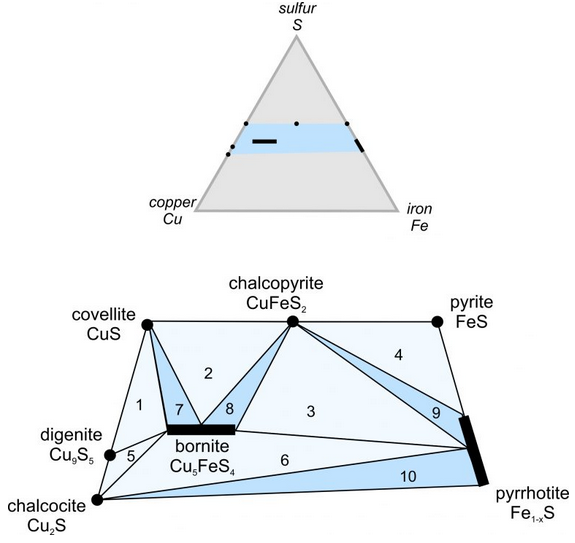
We can use a triangular diagram to plot the compositions of copper-iron sulfide minerals in the same way we plotted feldspar, pyroxene, and other silicate compositions in Chapter 6. The small gray triangular diagram in the top of Figure 9.46 depicts the Cu-Fe-S system. The blue region in the center of the triangle is where compositions of the most important ore minerals plot. The quadrilateral below the triangle is an enlargement of that blue region.
The quadrilateral shows seven minerals. Bornite and pyrrhotite, which have variable compositions appear as bars that show the variation. Different specimens of bornite may contain different amounts of copper and iron, and so bornite plots as a horizontal bar. Pyrrhotite contains variable amounts of iron and sulfur and so plots as a bar pointing at the iron and sulfur corners of the triangle. The other minerals do not vary much in composition and so appear as dots. The phase rule (discussed in Chapters 4 and 8) tells us that the number of minerals that can coexist is generally quite small for simple chemical systems. This triangle depicts a simple system with only three chemical components. Tie lines and triangles on the quadrilateral show minerals that may be found together. If, for example, a rock has composition that plots where the number 3 is, it will contain bornite, chalcopyrite, and pyrrhotite.
Cu-Fe sulfide mineralogy is complex because many minerals have similar compositions. The quadrilateral shows 10 triangular fields (numbered 1 through 10). Those in light blue are 3-mineral fields – any composition that plots within them will contain three sulfides. The darker blue triangles only connect two minerals; they are 2-mineral fields. Compositions that plot within them will contain only two sulfides. The assemblage present in a specific deposit depends on the Cu:Fe:S ratio. In Fe-poor ore deposits, for example, sulfide assemblages will include covellite, digenite, or chalcocite, but not pyrite or pyrrhotite. Diagrams such as the one in this box (Figure 9.46) are useful ways to describe complex mineral relationships without words, and we use them to predict and interpret ore deposit mineralogy.


