9.2.1: Native Elements- Metals, Semimetals, and Nonmetals
- Page ID
- 18487
Native elements have high value because they may require no processing before being used in manufacturing, as currency, or for other purposes. The first metals ever used by humans were native minerals. Only later did humans develop refining techniques for the extraction of elements from more complex minerals. We conveniently divide native elements into metals, semimetals, and nonmetals based on their chemical and physical properties. The table to the right includes the most common minerals of each group.
Within the metal group, the principal native minerals are gold, silver, copper, and platinum. These four minerals all contain weak metallic bonds. Gold, silver, and copper have further commonality in their chemical properties because they are in the same column of the periodic table. Gold and silver form a complete solid solution; we call compositions containing both gold and silver electrum. But, because copper atoms are smaller than gold and silver atoms, solutions are limited between copper and the precious metals. Native gold, silver, and copper may contain small amounts of other elements. For example, native copper frequently contains arsenic, antimony, bismuth, iron, or mercury. Native platinum is much rarer than gold, silver, or copper. It typically contains small amounts of other elements, especially palladium. The native semimetals arsenic, antimony, and bismuth are also rare.
| Native Metals | |
| gold | Au |
| silver | Ag |
| copper | Cu |
| platinum | Pt |
| Native Semimetals | |
| arsenic | As |
| antimony | Sb |
| bismuth | Bi |
| Native Nonmetals | |
| graphite | C |
| diamond | C |
| sulfur | S |
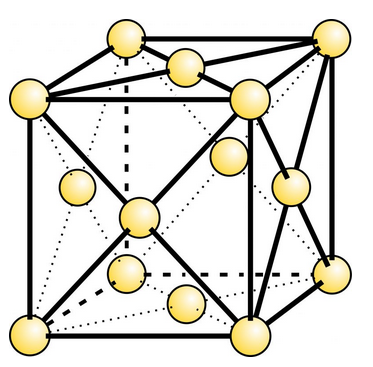
Native copper, gold, silver, and platinum have atomic structures with atoms arranged in a cubic pattern (Figure 9.23). Iron does, too, although native iron is rare, except in meteorites, and the atomic arrangement in native iron is not quite the same as in the other metals. Nonetheless, euhedral crystals of any of these minerals may be cubic or, as we will explain in the next chapter, octahedral. More typically, however, these minerals crystallize in less regular shapes. Native zinc, a very rare mineral, has a hexagonal atomic arrangement and so forms crystals of different shapes. The photos below (Figures 9.24 through 9.29) show examples of native gold, silver, copper, platinum, antimony, and sulfur.
Gold, sometimes mined as nuggets or flakes (see the example in Figure 9.17), is also found as wires or scales. Large, visible specimens, like the one seen below in Figure 9.24 are, however, unusual. Most gold and other precious metal ores contain very fine subhedral metal grains, often microscopic. Silver sometimes occurs in a wire-like or arborescent (tree-like) form (Figure 9.25). It also easily tarnishes and so has a gray color in this photo. Most bedrock gold and silver deposits are in quartz-rich hydrothermal veins. Pyrite (fool’s gold) and other sulfides are often associated with native gold and silver in these veins. Besides hard-rock deposits, gold and silver are also found in placers (accumulations in river, stream, or other kinds of sediments), and native silver is found in several other types of deposits. Box 9-3 (below) describes the Witwatersrand gold deposits, the largest gold deposits in the world. Section 9.3.3.1, later in this chapter, gives more detailed information about placers.






Native copper occurs in a variety of ore deposits associated with mafic volcanics and in some sandstones. Copper is found as branching sheets, plates, and wires, and as massive pieces. In Figure 9.26, it is in a discontinuous sheet that has partially altered to malachite, copper carbonate. We mine native platinum primarily from ultramafic igneous rocks, but platinum is also found in placers – Figure 9.27 shows examples placer platinum nuggets. Platinum is also a secondary product of Cu- or Ni-sulfide refining. Native antimony (in Figure 9.28), is rarely pure. It is usually in solution with arsenic and may contain small amounts of other metals. Untarnished specimens are metallic and silvery, but antimony typically tarnishes to a gray color as seen in this photo.
Graphite, diamond, and sulfur are examples of nonmetallic native elements. Figure 9.29 shows an example of native sulfur. Figure 3.49 (Chapter 3) contains another photo. Sulfur deposits are associated with volcanoes, often concentrated at fumaroles. Sulfur is also found in veins in some sulfide deposits and in sedimentary rocks where it is found with halite, anhydrite, gypsum, or calcite. Native sulfur deposits only account for about half the world’s sulfur supply. Most of the rest is separated from sulfides during processing to recover metals.
Both graphite and diamond consist only of carbon. We discussed the nature of the two minerals in Chapter 3. Graphite is common as a minor mineral in many kinds of metamorphic rocks, including marbles, schists, and gneisses. The origin of the carbon is usually organic material in the original sediments. Graphite also occurs in some types of igneous rocks and in meteorites. Diamond only forms at very high pressures associated with the lowermost crust or mantle of Earth. We mine it from kimberlite pipes, where rapidly moving, sometimes explosive, mafic magmas have carried it up to the surface. After formation, diamond sometimes concentrates in river and streambeds where we mine it from placer deposits. Although some diamonds are of gem quality, most are not. We call lower-quality diamonds industrial diamonds or bort (if the diamonds are small and opaque). See section 9.2.4, later in this chapter, for more information about diamonds and other gems.
The Witwatersrand Gold Deposits

Gold occurs in many different ore deposits. The world’s largest, the Witwatersrand deposit of northeastern South Africa, dominates world production. 40% of all the gold ever mined came from Witwatersrand ores. Figure 9.30 shows an example of very high-grade ore from the Blyvooruitzicht Gold Mine of that region. The yellow grains are gold, and the black material is uraninite. This ore, like many Witswatersrand ores, is quite radioactive.
The Witwatersrand deposits are paleoplacer deposits, meaning that they were placers when originally deposited. They occur in an area about 100 km by 40 km. The origin of the Witwatersrand deposits is a bit of a mystery. Placers form when hard-rock deposits are eroded, and sedimentary processes concentrate ore. Yet today we know of no hard-rock gold deposits of sufficient size to account for the volume of the Witwatersrand placers.
The Witwatersrand gold prospects were discovered in 1852, but the discovery was kept secret. It was not until 1886 that significant production began. A booming mining industry led to the rapid growth of Johannesburg, a central town in frontier South Africa. Within a decade, Johannesburg was the largest city in the country. When miners reached a zone of pyrite in 1889, the mining slowed because it was not known how to extract gold from sulfides at the time. Subsequently, John MacArthur, Robert Forrest and William Forrest, three Scotsmen working for the Tennant Company in Glasgow, developed a dissolution process involving cyanide that would extract gold from sulfides. So, Johannesburg flourished once more.


