7.4.4: Sulfate Minerals
- Page ID
- 18695
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)| Sulfate Minerals | |
| gypsum anhydrite barite celestite anglesite |
CaSO4•2H2O CaSO4 BaSO4 SrSO4 PbSO4 |
Mineralogists have described more than 100 sulfate minerals. They fall into two main groups: those that contain water (hydrous sulfates) and those that do not (anhydrous sulfates). Gypsum (CaSO4•2H2O) is the only common hydrous sulfate. Less common species include chalcanthite (CuSO4•5H2O), epsomite (MgSO4•7H2O), and antlerite Cu3SO4(OH)4. Examples of anhydrous sulfates include anhydrite (CaSO4), barite (BaSO4), celestite (SrSO4), and anglesite (PbSO4). Many others are known, but most are rare.
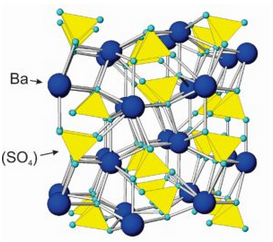
The drawing in Figure 7.41 shows how atoms are arranged in barite (BaSO4). Sulfur atoms bond to four oxygen, creating sulfate tetrahedra with composition (SO4)2-. The tetrahedra are tightly bonded and similar, in some ways, to the (SiO4)4- groups that characterize silicates. Sulfate tetrahedra, however, do not polymerize. Ba2+ cations alternate with anion sulfate and bond to oxygen at tetrahedral corners. The atomic arrangements are the same in the other anhydrous sulfates, with Ca2+, Sr2+, and Pb2+ substituting for Ba2+ in anhydrite, celestite, and anglesite, respectively. Besides the minerals listed in the blue box, at least a dozen other, but rare sulfates exist. Some are listed in the table of evaporite minerals earlier in this chapter.


Gypsum, hydrated calcium sulfate, is the most common sulfate mineral. It is found in thick evaporite deposits, in hydrothermal veins, and as precipitates from surface or subsurface waters, hot springs, or volcanic gases.
Anhydrite, with a composition equivalent to gypsum lacking H2O, is an evaporite mineral found in sedimentary rocks associated with sedimentary basins. It commonly found with gypsum, and alters to gypsum over time. Anhydrite may be in thick layered rocks that also contain halite and carbonate minerals.
Common gypsum is white to grey, and translucent, but just about all colors are known. Normal coarse crystals of gypsum form blades or tabs. If translucent, we call them selenite (Figures 7.42 and 7.43). Selenite commonly twins, sometimes forming characteristic swallow tail or fishtail twins. These two types of twinning are very similar and often not distinguished from each other. Figure 7.43 shows two examples of swallowtail twins. Another example is seen in Figure 4.39 (Chapter 4).


Satin spar is an especially fibrous variety of selenite (Figure 7.44). Satin spar is often opalescent and is sometimes used in jewelry, although it is soft and not very durable. Sometimes gypsum crystals form a flowery cluster called a desert rose (Figure 7.45). The specimen in Figure 7.45 is 47 cm across! In caves gypsum may form gypsum flowers, similar in many ways to desert roses. Alabaster is very fine grained gypsum that people sometimes carve or polish for building stone or for art.

The spectacular photo in Figure 7.46 shows a geologist in Mexico’s Cueva de los Cristales (Cave of Crystals). The cave contains some of the largest mineral crystals in the world – up to 12 meters long. These crystals are selenite, the translucent form of gypsum. Gypsum is moderately water-soluble, so it is one of a relatively small number of common minerals that precipitate from natural water, often redissolve, and later reprecipitate somewhere else. Most natural gypsum crystals are centimeters in size or smaller. However, extremely large crystals, such as those shown in the photo above, exist in several places around the world.
Gypsum: Ingredient of Plaster and Drywall

Plaster can be made from different mineral materials. Early Romans used a lime-based plaster, but plaster of Paris (Figure 7.47), which is gypsum-based plaster, was introduced around 1254 and is widely used today. At the time, the best and most productive gypsum quarries were in Montmartre, a section of Paris. Modern plasters and sheetrock both contain plaster of Paris. Manufacturers produce it by calcining (grinding and heating) gypsum (CaSO4 ∙2H2O) to reduce its water content. Most plasters contain about 25% of the gypsum’s original water. Complete dehydration of gypsum would produce anhydrite (CaSO4), which is not useful as a plaster because it does not recombine easily with water. However, when plaster of Paris is mixed with water, reaction occurs quickly, giving off heat and promoting drying and hardening.
Drywall (also known as sheet rock) is composed of gypsum with paper front and backing. A number of different additives may be mixed with the gypsum to promote desired properties, including strength, resistance to mildew, and reduced water absorption. Drywall construction replaced the more classic lath and plaster walls in the mid 1900s.


Barite, barium sulfate, commonly occurs in hydrothermal deposits with copper, lead, and zinc minerals; it is often associated with anglesite and celestite. Barite is also found in hot spring deposits, and in some evaporite deposits. The cluster of clear to light-blue bladed crystals in Figure 7.48 is typical for barite. And, barite sometimes occurs as concretions in sediments and sedimentary rocks, and sometimes as desert roses (Figure 7.49), similar to desert roses made of gypsum.


Celestite (also called celestine), strontium sulfate, most often occurs in sedimentary rocks associated with gypsum, anhydrite, sulfur, or halite. Commonly it has a diagnostic light blue color as seen in the photo here (Figure 7.50). Celestite is the most common strontium mineral. Strontianite (strontium carbonate) is the only other one of significance.
Some sulfates are common as minor, and rarely major, minerals in ore deposits – typically as replacements for primary sulfides. Anglesite (PbSO4), for example, forms during weathering or alteration as a replacement for galena (PbS). The specimen seen in Figure 7.51 contains light brownish anglesite that appears to have grown from the silver-gray galena.


