7.4.3: Carbonate Minerals
- Page ID
- 18694
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)| Carbonate Minerals | |
| Ca-Mg-Fe carbonates •calcite •aragonite •magnesite •siderite •dolomite •ankerite |
CaCO3 CaCO3 MgCO3 FeCO3 CaMg(CO3)2 CaFe(CO3)2 |
| Other •rhodochrosite •smithsonite •cerussite •strontianite •witherite •azurite •malachite •hydromagnesite •natron |
MnCO3 ZnCO3 PbCO3 SrCO3 BaCO3 Cu3(CO3)2(OH)2 Cu2CO3(OH)2 Mg5(CO3)4(OH)2•4H2O Na2CO3∙10(H2O) |
Mineralogists have identified more than 50 different carbonate species; all contain (CO3)2- groups but some contain other anions or anionic groups. The box lists some examples. The most important carbonates are Ca-Mg-Fe carbonates, especially calcite and dolomite, CaCO3 and CaMg(CO3)2, respectively. Less common rhodochrosite, smithsonite, cerussite, strontianite, azurite, and malachite sometimes form spectacular mineral specimens. These minerals are also important ore minerals of manganese, zinc, lead, strontium, and copper. The common carbonates have relatively simple compositions and include no hydroxyl groups or H2O. Some relatively rare species are more complex, however, and examples are at the bottom of the list.
Calcite is the most abundant carbonate mineral. It typically forms by organic processes or precipitation from oversaturated water. It also occurs in caves, where calcite forms stalactites and stalagmites. Calcite and other carbonate minerals also precipitate from hydrothermal waters (warm waters), especially in ore deposits and typically in veins. This is how most azurite and malachite, and some related ore minerals, are created.

Many carbonate minerals can have either an inorganic or an organic origin. Inorganic marine carbonate rocks form when either calcite or aragonite precipitate from ocean water. Marl may form when carbonates precipitate on lake or stream bottoms. In contrast, organic carbonate rocks form when algae, corals, clams and other organisms create structures and shells from CaCO3 dissolved in seawater. This may produce carbonate rocks directly, or residual shells, bones, and other material can accumulate to produce carbonate clastic rocks. The maze coral seen in this photo (Figure 7.24) builds its structure out of calcite.

Both calcite and dolomite are essential minerals in limestones and dolostones (limestones containing dolomite instead of calcite), and may be clasts in other kinds of sedimentary rocks. Some clastic rocks are held together by fine grained carbonate cement. Both calcite and dolomite are found in metamorphic rocks called marbles and in rare igneous rocks called carbonatites. The photo seen here (Figure 7.25) shows a marble made of coarsely crystalline calcite.

Many carbonate minerals are secondary minerals formed during weathering or diagenesis. For example, most dolomite is secondary and forms by reaction of calcite with Mg-rich water during diagenesis. Magnesite, (MgCO3), a related carbonate, forms as an alteration product of mafic and ultramafic rocks. Hydromagnesite (a hydrated equivalent of magnesite) forms by weathering of Mg-rich minerals, including olivine, serpentine, and others. Figure 7.26 shows hydromagnesite that has crystallized on the surface of basalt.
The photos below are examples of coarsely crystalline carbonate minerals. Figure 7.27 shows calcite crystals (Ca-carbonate) on top of siderite (Fe-carbonate). Figure 7.28 shows aragonite, which is a polymorph of calcite. The blue and green specimen in Figure 7.29 contains azurite and malachite. Both are Cu-carbonates and both are copper ore minerals. The pinkish crystals in Figure 7.30 are rhodochrosite (Mn-carbonate). Rhodochrosite is not always this color, but when it is, the color helps identify it. The azurite, malachite, and rhodochrosite come from a well-known mining district in western Colorado. The green crystals in Figure 7.31 are smithsonite (Zn-carbonate). Like rhodochrosite, smithsonite comes in various colors, but this green color is typical. The last photo (Figure 7.32) shows crystals of dolomite (Ca-Mg carbonate) on top of talc.






Unfortunately, most mineral specimens are not as well formed or beautiful as suggested by the photos above. So, below are three more typical examples of calcite. The calcite specimen on the left (Figure 7.33, below) is massive; it is difficult to pick out distinct crystal shapes. The blue calcite in Figure 7.34 and the translucent calcite in Figure 7.35 are cleavage fragments. Calcite cleaves easily into rhombohedral shapes like the ones seen here, and the flat surfaces are cleavage planes, not crystal faces. Blue calcite is rare but translucent calcite is not. Rhombohedral cleavage fragments are exceptionally common.



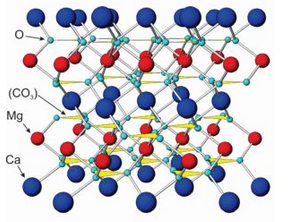
The atomic arrangements in calcite and dolomite, the most common carbonates by far, contain (CO3)2- groups alternating with Ca2+ or Mg2+. In the model of dolomite seen here (Figure 7.36), the blue atoms are calcium, the red ones magnesium, and the small aqua atoms are oxygen. Carbonate (CO3)2- groups are shown as yellow triangles. Other carbonates have similar structures. They all contain divalent anion carbonate groups and divalent cations such as Ca2+, Mg2+, Fe2+, Mn2+, or Zn2+. (Figure 7.36 is not to scale; the size of the carbonate groups is actually larger tham Ca2+ and Mg2+.)
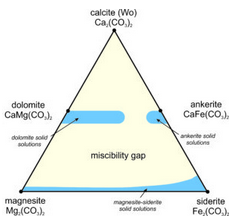
We can plot the compositions of Ca-Mg-Fe carbonates on a triangular diagram (similar to that used for pyroxenes in the previous chapter). Figure 7.37 shows, in blue, the ranges of stable carbonate compositions at low temperature; the stable compositions are limited. A large miscibility gap exists between the Ca-bearing carbonates and the Ca-free ones. It is similar to the gaps between wollastonite, clinopyroxene, and orthopyroxene we have already seen. And, the dolomite-ankerite series does not extend all the way across the diagram. As with the pyroxenes, the size of the miscibility gaps varies with temperature.
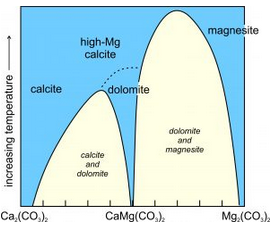
Figure 7.38 shows the simplfied schematic solvus relationships between calcite (CaCO3) and magnesite (MgCO3), equivalent to the left side of the triangle above. (It is simplified because melting and decarbonation reactions occur at high temperatures but are not shown.) We can see that the solvi narrow as temperature increases. At the highest temperatures on the diagram, calcite and high-magnesium calcite are stable. They are both Ca-Mg solid solutions of variable composition. Magnesite, an Mg-Ca solid solution, is stable too. At somewhat lower temperatures, high-Mg calcite changes into dolomite by an order-disorder transformation. The miscibility gaps (in yellow) at low temperature, mean that most carbonate compositions will unmix into calcite and dolomite, or dolomite and magnesite, solid solutions. Calcite may contain some extra magnesium, but the other two minerals will be close to end member composition. Calcite sometimes develops exsolution textures that we can see with a thin section and a petrographic microscope.
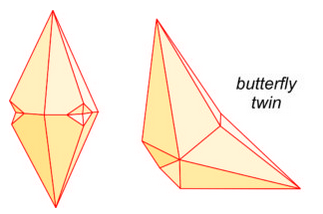

Calcite crystals twin by several different twin laws. The drawings seen here (Figure 7.39) show two common twin appearances. The drawing on the right is called a butterfly twin, and the photo in Figure 7.40 shows a good example of this kind of twinning. Calcite also commonly contains microscopic deformation twins that we can only see with a petrographic microscope.


