6.4.8: Amphiboles
- Page ID
- 18921
| Amphiboles (K,Na)0-1(Ca,Na,Mg)2(Mg,Fe,Al)5(Si,Al)8O22(OH)2 |
|
| Most important end members | |
| tremolite ferroactinolite anthophyllite cummingtonite grunerite glaucophane |
Ca2Mg5Si8O22(OH)2 Ca2Fe5Si8O22(OH)2 Mg7Si8O22(OH)2 Mg7Si8O22(OH)2 Fe7Si8O22(OH)2 Na2Mg3Al2Si8O22(OH)2 |
Amphiboles and pyroxenes are closely related minerals that commonly coexist. Both are chain silicates, but the atomic arrangement in amphiboles is more complex than in pyroxenes. Like pyroxenes, amphibole chemistry is highly variable and yields many different end member formulas. Just a few are listed in the blue box. Also, like the pyroxenes, amphiboles fall into two main series: the orthoamphibole series and the clinoamphibole series. Note that, in the box we have listed Mg7Si8O22(OH)2 two times. Anthophyllite is an orthoamphibole and cummingtonite is a clinoamphibole; the two are polymorphs. The lengthy generic formula at the top of the blue box describes hornblende, the most common kind of amphibole. The formula is complicated, but really does not reflect all possible compositional variations. Besides the elements listed, Mn and Ti are major components in some amphiboles, and F or Cl can replace (OH) in others. Note that hornblende contains variable amounts of K or Na (indicated by the subscript “0-1″) that are missing from all the other amphiboles listed in the box.
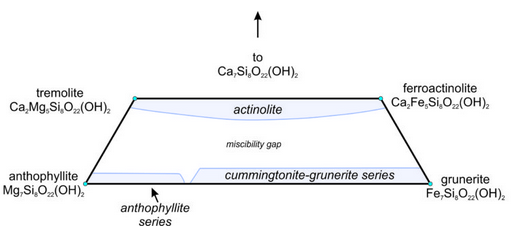
Figure 6.82 shows an amphibole quadrilateral, similar to the pyroxene quadrilateral we saw before. The quadrilateral contains a large miscibility gap between the calcic amphiboles and the calcium-poor amphiboles, analogous to the one between clinopyroxene and orthopyroxene. The quadrilateral includes the compositions of all Ca-Mg-Fe amphiboles. Along the base of the diagram, the figure is complicated because Ca-free amphiboles form two distinct series, having different atomic arrangements: the anthophyllite series and the cummingtonite-grunerite series.

We call the most Mg-rich amphiboles anthophyllite or, if they contain appreciable amounts of iron, ferroanthophyllite. Figure 6.83 shows a group of anthophyllite needles in a 4 cm wide specimen. The cummingtonite- grunerite series contains other intermediate and Fe-rich amphiboles that are poor in calcium. We give aluminous anthophyllite (not shown in the amphibole quadrilateral) the name gedrite.



Calcic amphiboles can have any composition between an Mg end member (tremolite) and an Fe end member (ferroactinolite). We call compositions between actinolite. The left and center photos above, Figures 6.84 and Figure 6.85, show typical actinolite and tremolite. Actinolite is generally green, and tremolite is white or light colored. The bladed crystals form because of the chain nature of atomic arrangements.

The amphibole quadrilateral depicts variations in Ca, Mg, and Fe content well, but many amphiboles, especially hornblendes, contain K, Na, Al, Ti, and other elements in significant amounts. Additionally, hornblende, the most common amphibole, is a complex mineral that contains many possible elements. It does not have the same number of atoms in its formula as quadrilateral amphiboles. Although many amphibole varieties have specific names, without chemical analyses, telling different amphiboles apart is difficult. So, the name hornblende is commonly used to refer to any black amphibole. The photo in Figure 6.86 shows three pieces of massive hornblende. This photo (Figure 6.87) shows an outcrop of metamorphic rock called amphibolite. All the dark mineral grains are hornblende.
Many amphibole end members have analogs in the pyroxene group; we compare some of the more important end members in the tables below.
|
|
||||||||
Amphiboles are important and essential minerals in many kinds of igneous rocks. For example, hornblende is widespread. It is common in granitic rocks but is even more common in rocks of intermediate to mafic composition. Amphiboles also exist in many metamorphic rocks, including marbles and metamorphosed mafic igneous rocks. They are especially common in high-temperature amphibolites like the one seen above in Figure 6.87. Amphibolites always contain amphibole and plagioclase, and generally other minerals including biotite, epidote, or garnet.

Figure 6.88 shows glaucophane, a blue sodic amphibole, that has the general formula Na2Al2Si8O22(OH)2. Its presence is often associated with rocks formed in subduction zones under high pressures and moderate temperatures. Other Ca-free amphiboles (anthophyllite, gedrite, cummingtonite, and grunerite) are found in metamorphic rocks and occasionally in extrusive igneous rocks. Tremolite is common in high-temperature marbles. As with some other silicates found in marbles, identifying it may be difficult because of its inconspicuous white color. See Figure 6.85, above.


