6.4: Silicate Minerals
- Page ID
- 18730
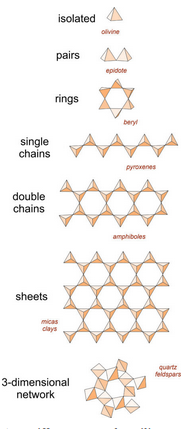
Silicate minerals dominate igneous rocks because silicon and oxygen are the most common elements in magmas. So, in the following discussion we systematically consider the important silicate minerals and groups.
Recall from Chapter 1 that the fundamental building block in silicate minerals is an (SiO4)4- tetrahedron with oxygen at the corners and silicon in the center. Individual tetrahedra bond to other tetrahedra or to cations to make a wide variety of atomic arrangements. The top drawing in this chart (Figure 6.24) shows a single isolated tetrahedron.
Sometimes aluminum substitutes for silicon, so silicate minerals may contain both (SiO4) and (AlO4) tetrahedra. A key property of both silicon and aluminum tetrahedra is that they can share the oxygen anions (O2-) at their corners. When they do this, they form polymers that are various kinds of rings, chains, sheets, or three-dimensional arrangements. Figure 6.24 shows some of the possibilities. (For a slightly different view, see Figure 1.33 in Chapter 1).
In some minerals, (SiO4)4- tetrahedra are not polymerized. They do not share oxygen atoms, and instead are joined together by bonds to other cations. These minerals, called isolated tetrahedra silicates, or island silicates, include most importantly minerals of the olivine group.
In a few minerals, especially minerals of the epidote group, tetrahedra join to form pairs. These are the paired tetrahedral silicates, sometimes called sorosilicates or butterfly silicates. In the pyroxenes and other single chain silicates, tetrahedra link to create zigzag chains. The amphibole minerals are double chain silicates. Micas and clays are sheet silicates. In sheet silicates, tetrahedra share three of their four oxygen with other tetrahedra, creating sheets and minerals with layered atomic arrangements.
Finally, the feldspars and quartz are examples of network silicates, sometimes called framework silicates, in which every oxygen is shared between two tetrahedra, creating a three-dimensional network. The drawing in Figure 6.24 does a poor job of showing this arrangement but the key characteristics are that all oxygen atoms are shared between two tetrahedra and the overall structure is about the same in all directions. The most important framework silicates are quartz and other SiO2 minerals, and the feldspars. In the feldspars, and a related group of minerals called feldspathoids, alkali and alkaline earth elements – mostly Na, K, or Ca – occupy large sites between tetrahedra.
We will start our review of silicate minerals by looking at the SiO2 polymorphs, the feldspars, and the feldspathoids, and then work our way upwards in the figure above, towards minerals with less polymerization.


