6.4.6: Chain Silicates
- Page ID
- 18919
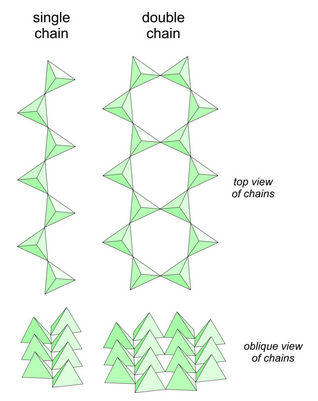
In micas, and other sheet silicates, silicon tetrahedra are polymerized to form sheets. In chain silicates, the tetrahedra polymerize to form chains. This is done in two ways, shown in Figure 6.70. Single chain silicates have tetrahedra that zig-zag back and forth. Double chain silicates also involve tetrahedra that zig-zag back and forth, but the chains come in pairs, and an oxygen anion, called a bridging oxygen, links chains together. A few relatively obscure minerals have chains that are wider and more complex than double chains – the are part way to being sheet silicates. Pyroxenes and amphiboles are the two most important groups of minerals in the chain silicate subclass. In fact, they are almost the only minerals in the subclass.
All chain silicates contain single or double chains of tetrahedra linked by cations. Pyroxenes, and their cousins pyroxenoids, are both single chain silicates and have closely related atomic arrangements. Amphiboles, such as tremolite or hornblende, are double chain silicates. Because two oxygen are shared with other tetrahedra, pyroxenes have formulas that contain (SiO3) or (Si2O6). Less obvious, but equally valid, is that amphibole formulas contain (Si8O22). In both pyroxenes and amphiboles, however, Al may substitute for significant amounts of Si.
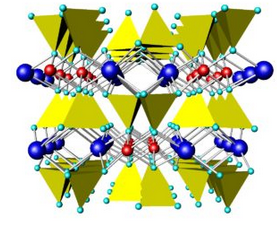
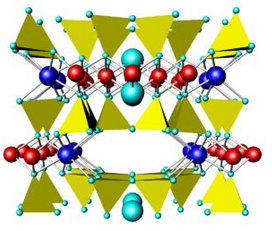
Figures 6.71 and 6.72 above compare the atomic arrangements in diopside and tremolite. Diopside is a Ca-Mg pyroxene (single chain), and tremolite is a Ca-Mg amphibole (double chain). In both figures, the view is down the chain direction; the yellow tetrahedra are the (SiO4) units that form the chains. In pyroxenes, the linking cations (blue and red in these figures) occupy two distinct kinds of sites between the chains; in amphiboles, they occupy three kinds of sites between the chains. In both pyroxenes and amphiboles, two interchain sites (blue) are significantly larger than the others. In diopside and tremolite, the large sites contain Ca and the smaller sites contain Mg. The large light blue spheres in the tremolite structure are hydroxyl (OH) groups. Compare these figures with the one above (Figure 6.70) to get a different perspective.
Pyroxenes are anhydrous minerals having simple formulas compared with amphiboles, all of which are hydrous. Still, the similarity between the structures of pyroxenes and amphiboles results in some similarity in physical properties. For example, unless cleavage is visible, it can be difficult to distinguish dark-colored pyroxenes from dark-colored amphiboles.


