6.4.2: SiO2 Polymorphs
- Page ID
- 18731
Silica Minerals
SiO2
α-quartz, β-quartz, stishovite, coesite, tridymite, cristobalite

Quartz, like many other minerals, is polymorphic. Mineralogists and chemists have identified more than 10 different silica (SiO2) polymorphs, but some do not occur as minerals. We briefly looked at the most common of these polymorphs in Chapter 4. All the silica minerals (except stishovite) are framework silicates; the differences between the minerals are the angular relationships between the tetrahedra that comprise them. Figure 6.25 shows the atomic arrangement in cristobalite, one of the high-temperature polymorphs. The blue spheres are oxygen atoms and silicon atoms are at the centers of every gray tetrahedron. For some spectacular scanning electron microscope images of several silica polymorphs, see Figure 12.35 in Chapter 12.


Common quartz, more properly called low quartz (because it has lower symmetry than high quartz), is the only polymorph stable under normal Earth surface conditions, but it has many different appearances. The anhedral specimen seen in Figure 6.26 has a very common look. But euhedral crystals, such as those shown in Figure 6.27, are also common. Some of the different quartz varieties have specific names, such as milky quartz, rose quartz, Herkimer diamond, amethyst, and citrine. Photos of these were in previous chapters, including:
- Figures 1.8 (Chapter 1) shows a cluster of clear quartz crystals
- Figure 3.4 (Chapter 3) contains a photo of subhedral rose quartz
- Figure 3.32 (Chapter 3) shows a Herkimer diamond
- Figure 3.44 (Chapter 3) show purple amethyst and orange citrine
- Figure 3.61 (Chapter 3) is a photo of anhedral milky quartz
- Figure 4.17 (Chapter 4) shows amethyst in a geode
Silica Polymorph Stability
For several decades, petrologists have understood that different silica polymorphs occur in different geological settings because they are stable under different pressure-temperature conditions. This phase diagram (Figure 6.28) shows the stability relationships of some SiO2 polymorphs. The horizontal axis is temperature. The vertical scale on the left gives pressures in gigapascals (GPa), and the scale on the right shows the depths in Earth corresponding to those pressures.
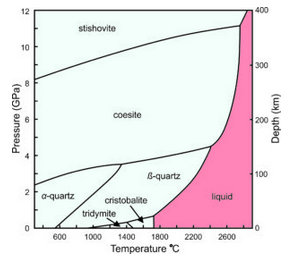
Pressure-temperature (P-T) phase diagrams such as the one seen here show which mineral is stable for any combination of P-T. Solid lines divide pressure-temperature space into stability fields where different polymorphs are stable: stishovite, coesite, α-quartz, β-quartz, tridymite, and cristobalite. For example, at 1400 ̊C and 6 GPa, coesite is the stable polymorph. Low quartz (α-quartz) is the stable phase at low temperature and pressure, including normal Earth surface conditions (which would plot way off to the left on the bottom of this diagram). Low quartz is therefore the most common polymorph. If all rocks maintained and stayed at equilibrium, we would have no samples of any other silica polymorphs to study. However, the other polymorphs sometimes persist and exist metastably at Earth’s surface. (Although given enough time, they usually change into low quartz.)
Stishovite and coesite are dense minerals, only stable at very high pressures – pressures not normally encountered on or in Earth’s crust. They are usually associated with meteorite impact craters. Tridymite and cristobalite only exist in certain high temperature silicic volcanic rocks. They require temperatures greater than 900 °C to form. Although not shown in this diagram, just like quartz, tridymite and cristobalite have both high- and low-symmetry polymorphs.
The red field in the phase diagram above (Figure 6.28) shows conditions where silica melts. Melting temperature is greatest at high pressure, and is different for the different polymorphs. So, with cooling, molten SiO2 will crystallize to form stishovite at high pressure, coesite at somewhat lower pressure, β-quartz at still lower pressure, and cristobalite at 0.0001 GPa (equivalent to atmospheric pressure at Earth’s surface). Consider what happens when a volcano erupts and silica-rich magma cools. If stability is maintained, cristobalite will crystallize at about 1725 °C. With further cooling, it will turn into tridymite at about 1460 °C, then into β-quartz at about 950 °C, and become α-quartz at 573 °C. This happens most of the time but occasionally metastable polymorphs can be found in volcanic rocks.
Quartz in Rocks
Essential minerals are minerals that must be present for a rock to have the name that it does, and quartz is an essential mineral in silicic and intermediate igneous rocks, many sediments, and many metamorphic rocks. Quartz is not normally found in mafic igneous rocks because crystallization of mafic minerals such as olivine or pyroxene generally consumes all silica that is available, so there is none left over to form quartz.

Granites contain essential quartz. In silicic plutonic rocks such as granite, quartz is always associated with K-feldspar, commonly in a mosaic pattern similar to what is seen in this photograph (Figure 6.29). The largest of the grains in this view are pinkish K-feldspar about 1 cm across. Quartz is glassy gray. White plagioclase and black biotite are also present.

Quartz is also an essential mineral in sandstone and some other sedimentary rocks. Quartz is the only mineral present in some sandstones or cherts. But, sandstone may also contain significant amounts of other minerals including feldspar or clay, and sometimes pebbles or rock fragments. Figure 6.30 shows an arkose, a feldspar-containing variety of sandstone, composed of gray quartz, pinkish-orange feldspar, and some obvious quartz pebbles up to 5 mm across.
Quartz cannot exist in rocks containing corundum (Al2O3), because the two minerals would react to form an aluminosilicate mineral of some sort. It cannot exist in rocks containing feldspathoids (leucite, nepheline, or analcime) because quartz and feldspathoids react to give feldspars. For similar reasons, quartz is absent or minor in many alkali-rich igneous rocks and in rocks containing the oxide mineral spinel (MgAl2O4).


Quartz may crystallize from silica-saturated water. The photograph on the left above (Figure 6.31) shows white quartz veins cutting through altered granite in Kings Canyon National Park, California. The quartz formed when hot hydrothermal water infiltrated the rock along fractures before the rock was uplifted to the surface and eventually weathered. Temperature need not be high, however, for quartz to precipitate. For example, the amethyst (purple quartz) in the geode shown in Figure 6.32 precipitated from water at low temperature. Figure 4.17 (Chapter 4) shows another example of quartz in a geode.
Quartz Twins

Most quartz crystals are twinned, but the twinning can be impossible to see or easily overlooked. The drawings seen here (Figue 6.33) show the three major ways that quartz twins. Brazil twins and Dauphine twins are penetration twins, and Japanese twins are contact twins. All three are generally growth twins but can also form in other ways. Some quartz crystals exhibit more than one kind of twinning.

Brazil and Dauphine twins are distinguished by symmetry relationships between crystal faces of particular shapes, sometimes by identifying striations (fine lines on crystal faces that developed when the crystal formed), but sometimes are difficult to tell apart. Click on the crystal drawing (right) to see a 15 second video showing Dauphine twinning; the striations on the crystal faces are also apparent. The two large quartz crystals in Figure 6.27, earlier in this chapter, show an example of Japanese twinning.


