6.3.3: Disequilibrium
- Page ID
- 18729
If magma and minerals remain in equilibrium, different minerals crystallize at different temperatures – sometimes more than one at a time, and mineral compositions change as temperature decreases. Continuous reactions take place as elements move from magma into growing crystals. These relationships are orderly and predictable for a magma of any given composition. But, they are highly dependent on composition. Granite and gabbro, for example, do not crystallize the same minerals, nor do they crystallize at the same temperatures.
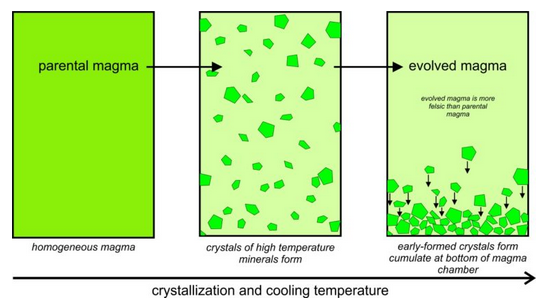
However, mineral crystals and magmas do not always remain in equilibrium during crystallization. Several things may cause disequilibrium. For example, as depicted in Figure 6.18, the first crystals that form may become separated from the rest of the magma if they settle to form a layer called a cumulate at the bottom of the magma chamber. The crystals, then, will not stay in equilibrium with the magma above them. As this happens, an original parental magma changes composition and becomes an evolved magma. Many minerals, including feldspars, pyroxenes, and oxides, can be found in thick, nearly single-mineral cumulates. And, even if a thick cumulate layer does not develop, the composition of the upper part of the magma chamber may not be the same as the lower part. When a melt gets separated from early formed crystals, we call the process partial crystallization, or fractional crystallization.

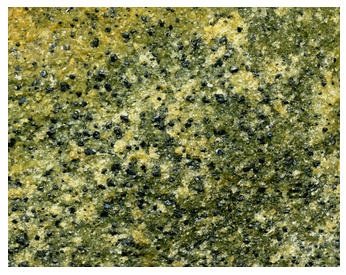
The photos above in figures 6.19 and 6.20 show examples of cumulates. The photo on the left (Figure 6.19) shows dark layers of chromite (chrome-iron oxide) that settled to the bottom of a magma chamber in the Bushveld Complex of South Africa. The light material around the chromite layers is mostly the plagioclase. The Bushveld region is one of the world’s foremost mining regions, in part because the natural concentration of chromite makes it easy for the ore to be mined and produced for industrial consumption. Figure 6.20 shows a cumulate from the Stillwater Complex (Montana) that contains both olivine (green) and chromite (black). Like the Bushveld, the Stillwater Complex sees significant mining activity.
Layered Mafic Intrusions
Mafic and ultramafic layered intrusions, often called layered mafic complexes, are rare but are found worldwide – generally in old continental interiors. The largest, the Bushveld Complex of South Africa, is more than 66,000 km2. Smaller layered intrusions, such as the Skaergaard Complex (Greenland), may be only 100 km2 or less.

In the United States, Montana’s Stillwater Complex accounts for most of the platinum group metals mined in the western hemisphere. The photo seen in Figure 6.21 shows some of the richest ore at Stillwater. The metallic minerals are pyrrhotite and chalcopyrite that contain significant amounts of platinum, palladium, osmium, and other generally rare elements.

Besides separation of crystals and melt, disequilibrium occurs for other reasons. Sometimes large mineral grains do not remain in equilibrium with a surrounding magma. For example, because diffusion of elements through solid crystals is slow, different parts of large crystals may not have time to maintain equilibrium compositions. In principle, mineral crystals should be homogeneous, but in zoned crystals, such as the tourmaline crystals seen in this photo (Figure 6.22), only part of the crystal remains in equilibrium with the melt as crystallization takes place. Consequently, different zones have different compositions (and sometimes different colors).
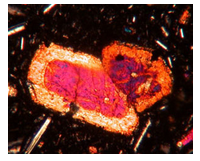
Marked chemical zonation often occurs if a magma begins to cool at one depth and then rapidly moves upward to cooler temperatures. The result is often a porphyritic rock with large zoned phenocrysts. The zones may be visible with the naked eye, for example the tourmaline in Figure 6.22, if color or textural variations mirror the compositional variations. Other examples of visibly zoned minerals include the tourmaline in Figure 4.13 (Chapter 4), and the fluorite in Figure 4.37 (Chapter 4). If the zoning is not visible to the naked eye, it may be visible when a crystal is viewed in thin section with a petrographic microscope . The thin section photo seen here (Figure 6.23) shows zoned clinopyroxene. The colors are artifacts and are not real mineral colors. Zoning can also be detected using a scanning electron microscope. See, for example, the electron microscope images of zoned plagioclase in Figure 4.38 (Chapter 4).


