3.5.3: Hardness
- Page ID
- 19171
Hardness is a mineral’s resistance to abrasion or scratching. We determine relative hardness (symbolized by H) using a scratch test: we try to scratch a surface of one mineral with an edge or corner of a second mineral. If a scratch or abrasion results, the first mineral is the softer. Absolute hardness is not quite the same as relative hardness. It is the measure of a material’s ability to resist permanent deformation. Although rarely done by mineralogists, values of absolute hardness may be determined in several ways; the easiest is to use an indenting tool similar to ones used to measure the hardness of steel. The indenting tool measures the force necessary to produce a permanent indentation in a flat surface.
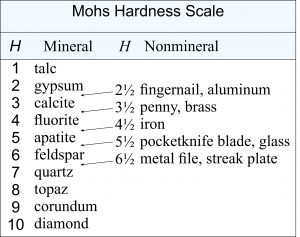
This table gives the relative hardness scale that is used by mineralogists. Based on ten well-known minerals, it is called the Mohs Hardness Scale, named after Austrian mineralogist Friedrich Mohs who developed it in 1812. The Mohs scale ranks minerals by their ability to scratch each other. The Mohs scale is related to absolute hardness but does not measure the same thing because resistance to scratching depends on additional factors besides the force needed to make an indentation.
If we compare the Mohs hardness scale with absolute hardness, we find that the Mohs scale is not linear and is close to being exponential. The hardnesses of the softest minerals are more similar than the hardnesses of the four hardest ones (quartz, topaz, corundum, diamond). Gypsum (H = 2) is only slightly harder than talc (H = 1), but diamond (H = 10) has a hardness five times greater than corundum (H = 9).

We can estimate relative hardness by conducting scratch tests to compare the hardness of an unknown mineral to the minerals in the Mohs hardness scale. Many labs are equipped with boxes of reference minerals for this purpose.
Alternatively, we can approximate hardness by comparing mineral hardness with the hardness of a fingernail, penny, pocketknife, glass, or several other common objects – the most commonly used are listed in the table above. Figure 3.72 shows gypsum being scratched with a fingernail. Gypsum, one of the softest minerals known, has a hardness of 2 on the Mohs hardness scale; fingernails have a hardness of about 2½. A penny has hardness of 3½, iron has hardness of about 4½, a pocketknife has hardness of 5½, and a metal file has hardness of 6½.
Scratch tests are often straightforward, but there can be complications. Mineral specimens may be too small or too valuable to scratch. Large samples may consist of many grains loosely cemented together so that scratch tests are not possible. Other samples may cleave or fracture when we perform tests. In still other cases, the results of scratch tests may be ambiguous, especially if we try to scratch a mineral with one that has the same, or nearly the same, hardness.
Most minerals have hardness greater than 2 and less than 7. The tables below list some examples of relatively common minerals that fall outside this range.
| Hardest Minerals | ||
| name | formula | hardness |
| cordierite | (Mg,Fe)2Al4Si5O18 | 7 |
| quartz | SiO2 | 7 |
| andalusite | Al2SiO5 | 7½ |
| zircon | ZrSiO4 | 7½ |
| beryl | Be3Al2Si6O18 | 7½ to 8 |
| spinel | MgAl2O4 | 7½ to 8 |
| topaz | Al2SiO4(F,OH)2 | 8 |
| chrysoberyl | BeAl2O4 | 8½ |
| corundum | Al2O3 | 9 |
| diamond | C | 10 |
| Softest Minerals | ||
| name | formula | hardness |
| talc | Mg3Si4O10(OH)2 | 1 |
| molybdenite | MoS2 | 1 to 1½ |
| graphite | C | 1 to 2 |
| pyrophyllite | Al2Si4O10(OH) | 1½ |
| covellite | CuS | 1½ to 2 |
| orpiment | As2S3 | 1½ to 2 |
| realgar | AsS | 1½ to 2 |
| gypsum | CaSO4•2H2O | 2 |
| stibnite | Sb2S3 | 2 |
| sylvite | KCl | 2 |
The hardness of a mineral relates to its weakest bond strength. So, because bonds are usually not the same in all directions in minerals, hardness may vary depending on the direction a mineral is scratched. In kyanite, for example, hardness varies from 4½ to 6½ depending on the direction of the scratch test. In most minerals, however, hardness is about the same in all directions. While the general relationship between hardness and bond strength is known, mineralogists have difficulty predicting hardness for complex atomic structures. For some simple ionic compounds, however, theoretical calculations match measurements well. Minerals with high density, highly charged ions, small ions, or covalent bonding tend to be hardest.


