3.5.2: Cleavage, Fracture, and Parting
- Page ID
- 19170
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Cleavage, fracture, and parting are three related terms that describe how a mineral crystal may break. The orientation and manner of breaking provide important clues about crystal structure and can be keys to mineral identification. If breaking produces planar and smooth surfaces, oriented in a particular way relative to a crystal’s atomic arrangement, we say that the mineral has cleavage.
If a mineral cleaves along one particular plane, a nearly infinite number of parallel planes are equally prone to cleavage. This is because of the repetitive arrangement of atoms in crystal structures. The spacing between cleavage planes is the repeat distance of atoms, on the order of angstroms (1Å = 10-10 m) for mineral crystals. The whole set of planes, collectively referred to as a cleavage, represents planes of weak bonding in the crystal structure. If cleavage produces mineral fragments with distinctive shapes, we use geometric terms such as cubic, octahedral, rhombohedral, or prismatic to describe the cleavage when appropriate. Overall, the direction and angular relationships between cleavages give valuable hints about atomic arrangements.
The vast majority of minerals exhibit cleavage, but the nature of cleavage is highly variable, and some minerals cleave much more easily than others. We use general terms to describe the quality of a cleavage, including: perfect, imperfect, distinct, good, fair, and poor. Although some minerals exhibit no cleavage, many cleave in more than one direction, but the qualities of the different cleavages may not be the same.
Some minerals contain only poorly developed cleavage. Others, like calcite, have perfect cleavage. Whether perfect or not, when present, cleavage can be a good property to help identify minerals. For example, some specimens of black pyroxene and hornblende may appear quite similar. But, pyroxenes have two cleavages at about 90o to each other, and hornblende has two cleavages at 60o to each other. Often we can see the cleavage angles in hand specimen, although sometimes it requires a hand lens.

Fracture is closely related to cleavage but is a general term that describes the way a mineral breaks or cracks in directions unrelated to weak atomic bonding. Minerals that are bonded with equal strength in all directions, such as quartz, have no cleavage, but instead fracture to form irregular surfaces. These minerals break along curved surfaces to form conchoidal fractures, similar to what happens when glass breaks. Figure 3.61 shows a sample of quartz displaying conchoidal fracture. Other kinds of fracture include even fracture, hackly fracture, splintery fracture, and others. The terms we use to describe fracture and cleavage are plentiful; the most commonly used terms are defined in the tables below.
space
| Terms Used to Describe Cleavage | |
| basal | well developed planar cleavage in one direction only; also sometimes called “platy” (micas) |
| cubic | three cleavages at 90̊ to each other (galena) |
| rhombohedral | three cleavages not at 90̊ to each other (calcite) |
| octahedral | four cleavages that produce 8-sided cleavage fragments (fluorite) |
| prismatic | multiple directions of good cleavage all parallel to one direction in the crystal (tremolite) |
| distinct | cleavage is present but may not be manifested as perfectly smooth planes |
| indistinct | cleavage is present but neither smooth nor regular |
| Terms Used to Describe Fracture (and example minerals) | |
| even | breaking to produce smooth planar surfaces (halite) |
| uneven / irregular | breaking to produce rough and irregular surfaces (rhodonite) |
| hackly | fracturing to produce jagged surfaces and sharp edges (copper) |
| splintery | forming sharp splinters (kyanite, pectolite) |
| fibrous | forming fibrous material (chrysotile, crocidolite) |
| conchoidal | breaking with curved surfaces as in the manner of glass (quartz) |
space
Micas, including muscovite and biotite, are the best examples of minerals with one excellent cleavage. The photo below (Figure 3.59) shows cleavage in biotite. Biotite can be easily broken into very thin sheets. Molybdenite, too, has only one direction of cleavage and breaks into sheets (see the molybdenite in Figure 3.40). The micas, molybdenite, and many other minerals that cleave into thick slabs or sheets have what we call basal cleavage.

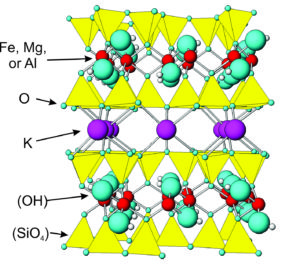
The ball and stick model in Figure 3.60 shows the atomic arrangement in micas. It is similar to the arrangement in all sheet silicates. The layered structure explains why micas cleave as they do. Micas cleave into sheets because the bonds to the potassium ions are very weak compared with all bonding in other directions.
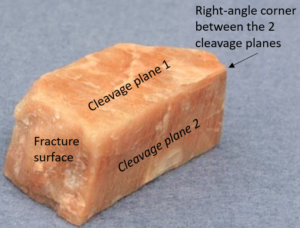
Feldspars, like the K-feldspar shown, have two cleavage directions. One is very good (meaning it is often easily observed) and the other better described as distinct (meaning it sometimes shows). The angle between the two feldspar cleavages is about 90o, which explains why the specimen in Figure 3.62 appears to have a square cross section. Cleavages in feldspar are, however, generally not as well developed as cleavages in minerals like mica and, so, may be hard to discern. Feldspars are typical – in ideal specimens the cleavages and angles between them can be easily seen. But, in many specimens, they cannot.

The photo seen in Figure 3.36 shows albite, another kind of feldspar. Hints of cleavage can be seen, but it is not obvious how many cleavages are present or what their angular relationships are. But, if you click on the photo of albite, you will get to a 3-dimensional photograph that you can rotate so you see the cleavages. Look closely and you will see that the angle between cleavages is just about 90o.
Some minerals with two cleavages, such as kyanite, easily break into long splintery pieces. Anthophyllite, too, sometimes forms bladed crystals. The photos below show typical blue blades of kyanite and a cluster of bladed anthophyllite crystals.


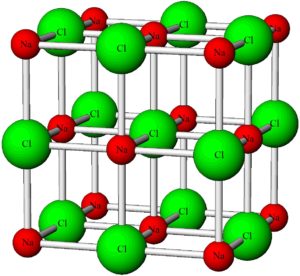
Other minerals, including halite and calcite, have three directions of cleavage. The ball and stick model below shows the atomic arrangement in halite. Atoms are evenly spaced and all bonds are perpendicular. Consequently, halite has cubic cleavage – three directions of cleavage at 90o to each other. In the blue/gray halite crystal below, the cleavages created a cube, and additional cleavage traces can be seen as fine cracks. Figures 3.2 and 3.10 also show halite. The cleavage cannot be seen in Figure 3.2 but is very clear in Figure 3.10


Like halite, calcite has three good cleavages. But, unlike halite, the cleavages are not perpendicular to each other. So calcite cleaves into shapes called rhombs. This photo shows a bunch of calcite cleavage fragments. Compare the shape of these fragments with the clear calcite shown in Figure 3.38. Besides calcite, dolomite and other carbonate minerals that belong to the rhombohedral carbonate group, cleave the same way. Some carbonates, such as aragonite and cerussite, however, are orthorhombic carbonates with three perpendicular cleavages.
Euhedral fluorite crystals are typically cubes, but sometimes they are octahedra like those shown in Figure 3.69 below. Fluorite is the most common example of a mineral that has four perfect cleavages; the cleavage directions are parallel to the octahedral faces. Many fluorite cleavage fragments are perfect octahedra, and many cubic fluorite crystals are missing corners because of the mineral’s cleavage. All fluorite is essentially CaF2 but color varies due to minor chemical impurities. Purple is the most common color, but the other hues in this figure are quite common too.

Click on the image below to explore a 3-dimensional model of cleavage in biotite, K-feldspar, and quartz. The biotite has perfect basal cleavage and the quartz shows conchoidal fracture. The feldspar cleavages is almost impossible to see.

Crystal faces and cleavage surfaces may be difficult to tell apart. In some minerals, principal cleavage directions are parallel to crystal faces, but in most they are not. A set of parallel fractures suggests a cleavage, but if only one flat surface is visible, there can be ambiguity. However, this problem is sometimes mitigated because crystal faces often display subtle effects of crystal growth. Twinning (oriented intergrowths of multiple crystals) and other striations (parallel lines on a face), growth rings or layers, pitting, and other imperfections often make a face less smooth than a cleavage plane, and give it lower reflectivity and a more drab luster.

The photo seen in Figure 3.71 is of a calcite crystal that shows visible striations on its crystal faces. The lines are artifacts of crystal growth and are not related to cleavage.
Some minerals exhibit parting, a type of breaking that is often quite similar to cleavage. Parting occurs when a mineral breaks along structural planes but, unlike cleavage, parting is not found in all samples of a particular mineral and does not repeat to form many parallel planes that are only a few angstroms apart. Several things can induce parting, perhaps most commonly it occurs because of twinning (when multiple crystals grow together and share atoms). Distinguishing parting from cleavage can, sometimes, be problematic.
For many good examples of mineral fracture and cleavage, watch the video linked below:
Video 3-6: Examples of mineral fracture and cleavage (8 minutes)
A good additional perspective on cleavage can be found at:


