4.5: Formation of Minerals
- Page ID
- 22620
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Minerals form when atoms bond together in a crystalline arrangement. In order for a mineral crystal to grow, the elements needed to make it must be present in the appropriate proportions, the physical and chemical conditions must be favorable, and there must be sufficient time for the atoms to become arranged.
Physical and chemical conditions include factors such as temperature, pressure, presence of water, pH, and the amount of oxygen available. Time is one of the most important factors because it takes time for atoms to move from place to place and become ordered. If time is limited, the mineral grains (with crystalline structure) will remain very small. The presence of water enhances the mobility of ions and can lead to the formation of larger crystals over shorter time periods.
Common Processes of Mineral Formation

- Crystallization from molten rock material (magma or lava). The majority of minerals in the crust have formed this way.
- Organic formation: formation of minerals by organisms within shells (primarily calcite) and teeth and bones (primarily apatite)
- Precipitation from aqueous solution (i.e., from hot water flowing underground, from evaporation of a lake or inland sea, or in some cases, directly from seawater).
- Weathering: during which minerals unstable at Earth’s surface (conditions of low temperature, low pressure, high moisture, and high oxygen levels) may be altered to other minerals.
- Metamorphism: formation of new minerals directly from the elements within existing minerals under conditions of elevated temperature and/or pressure.
These processes are responsible for the formation of the various mineral groups that compose the Earth’s crust. See the diagram below which illustrates the composition of the Earth’s crust by the minerals that compose it.
Crystallization from Magma and the Formation of the Silicate Minerals
Most of the minerals of Earth’s crust formed through the cooling of molten rock (magma or lava). Molten rock is very hot, typically on the order of 1000 \(^{\circ}\)C (1800 \(^{\circ}\)F) or more. Heat is energy and temperature is a measure of that energy. Heat causes atoms to vibrate and temperature measures the intensity of the vibration. If vibrations are very strong, chemical bonds will break and the pre-existing minerals in Earth’s crust and mantle will melt. Melting releases ions into a pool, forming a magma chamber. Magma is simply molten rock with freely moving ions. If magma is allowed to cool at depth or erupted onto the surface (then called lava), mineral crystals will form as temperature decreases.

The chemical composition of Earth’s crust is critical to the discussion of the dominant minerals to form from magma or lava, the silicate minerals. It is important to understand that not every location in the crust will have this composition. All rock is different and virtually no two rocks within the crust will have the exact same composition. The same also goes for magma. No two magma bodies will have the exact same composition. The magma will however, contain these elements in somewhat varying proportions depending on where exactly within the crust (or mantle) the magma was derived. What we can say is that the magma will be largely composed of silicon and oxygen with varying proportions of the remaining six elements plus other elements in trace amounts. The combination of these elements will form the different silicate minerals as the magma cools either deep within the Earth or near/at the surface.
Bowen’s Reaction Series
Crystallization of minerals from magma, or cooling lava, follows a very specific sequence. This sequence was first determined experimentally by the scientist Norman L. Bowen (1887-1956) at the Carnegie Institute in Washington, D.C. in the early 1900’s. This order is referred to as “Bowen’s Reaction Series” and represents the formation of the largest group of minerals, silicate minerals (see diagram below). This specific process of crystallization leads us to the formation of nine of “The Big Ten” minerals: olivine, pyroxene (augite), amphibole (hornblende), biotite, calcium-rich plagioclase (anorthite), sodium-rich plagioclase (albite), potassium-rich feldspar (commonly orthoclase), muscovite, and quartz.
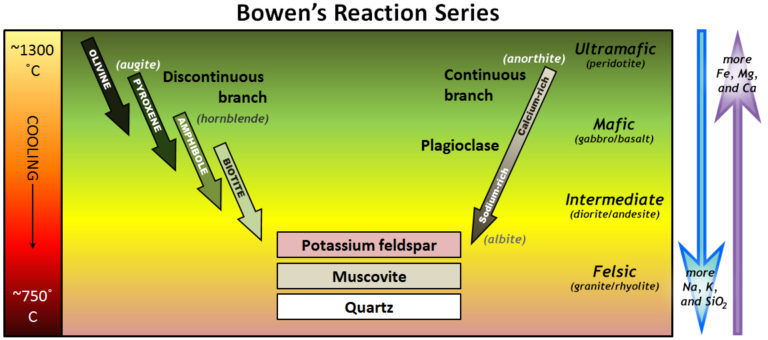
The left side of Bowen’s Reaction Series Diagram is labeled the “Discontinuous Branch” while the right side is labeled the “Continuous Branch.” On the left side, the minerals are most iron- and magnesium-rich at the top. Their crystal structure changes and iron and magnesium content decrease as temperatures drop. On the right side, the plagioclase minerals form in one continuous flow from calcium-rich at the top to sodium-rich at the bottom.
Beginning with the “Discontinuous Branch,” olivine forms from single silica tetrahedron ionically bonded to iron and/or magnesium for the crystalline structure. As the magma temperature cools and crystallization proceeds, the silica tetrahedron will link together (“polymerize”) to form chains, sheets and frameworks. This allows for the formation of the different minerals in progression along the Discontinuous Branch.

As the temperature drops, and assuming that some silica remains in the magma, the olivine crystals react (combine) with some of the silica in the magma to form pyroxene (commonly augite). As long as there is silica remaining and the rate of cooling is slow, this process continues down the discontinuous branch: olivine to pyroxene, pyroxene to amphibole (commonly hornblende), and amphibole to biotite.

At about the point where pyroxene (augite) begins to crystallize from the cooling magma, plagioclase feldspar also begins to crystallize on the continuous (right) side of Bowen’s Reaction Series Diagram. As cooling continues, plagioclase minerals retain their original framework silicate crystal structure. The core of the crystal begins calcium-rich and, once crystallization is complete, ends with a rim of sodium-rich plagioclase. See the photo below. Finally, if the magma is quite silica-rich to begin with, there will still be some left at around 750\(^{\circ}\) to 800 \(^{\circ}\)C, and from this last magma, potassium feldspar, quartz, and maybe muscovite mica will form.
Select play on the following animation to see how silicate minerals form from a cooling magma. Use the sliders to change the overall silica content (ultramafic to felsic) and cooling time. This is a very good visual representation of how the ions of the different common elements exist in magma and combine to form the different minerals found on Bowen’s Reaction Series as temperature decreases and the magma crystallizes.
[The webpage at https://www.mathieu-lessard.com/Geology/magma.html might be temporarily down or it may have moved permanently to a new web address.]
(Mathieu Lessard, with permission.)
Watch the following video which will introduce you to the silicate minerals:


