5.3: Sedimentary Rocks
- Page ID
- 6869
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Sedimentary rock is classified into two main categories: clastic and chemical. Clastic or detrital sedimentary rocks are made from pieces of bedrock, sediment, derived primarily by mechanical weathering. Clastic rocks may also include chemically weathered sediment. Clastic rocks are classified by grain shape, grain size, and sorting. Chemical sedimentary rocks are precipitated from water saturated with dissolved minerals. Chemical rocks are classified mainly by the composition of minerals in the rock.

Lithification and Diagenesis
Lithification turns loose sediment grains, created by weathering and transported by erosion, into clastic sedimentary rock via three interconnected steps. Deposition happens when friction and gravity overcome the forces driving sediment transport, allowing sediment to accumulate. Compaction occurs when material continues to accumulate on top of the sediment layer, squeezing the grains together and driving out the water. The mechanical compaction is aided by weak attractive forces between the smaller grains of sediment. Groundwater typically carries cementing agents into the sediment. These minerals, such as calcite, amorphous silica, or oxides, may have a different composition than the sediment grains. Cementation is the process of cementing minerals coating the sediment grains and gluing them together into a fused rock.

Diagenesis is an accompanying process of lithification and is a low-temperature form of rock metamorphism (see Chapter 6, Metamorphic Rock). During diagenesis, sediments are chemically altered by heat and pressure. A classic example is aragonite (CaCO3), a form of calcium carbonate that makes up most organic shells. When lithified aragonite undergoes diagenesis, the aragonite reverts to calcite (CaCO3), which has the same chemical formula but a different crystalline structure. In sedimentary rock containing calcite and magnesium (Mg), diagenesis may transform the two minerals into dolomite (CaMg(CO3)2). Diagenesis may also reduce the pore space, or open volume, between sedimentary rock grains. The processes of cementation, compaction, and ultimately lithification occur within the realm of diagenesis, which includes the processes that turn organic material into fossils.
Detrital Sedimentary Rocks (Clastic)
Detrital or clastic sedimentary rocks consist of preexisting sediment pieces that come from weathered bedrock. Most of this is mechanically weathered sediment, although some clasts may be pieces of chemical rocks. This creates some overlap between the two categories, since clastic sedimentary rocks may include chemical sediments. Detrital or clastic rocks are classified and named based on their grain size.
Grain Size
Detrital rock is classified according to sediment grain size, which is graded from large to small on the Wentworth scale (see figure). Grain size is the average diameter of sediment fragments in sediment or rock. Grain sizes are delineated using a logbase-2 scale [9; 10]. For example, the grain sizes in the pebble class are 2.52, 1.26, 0.63, 0.32, 0.16, and 0.08 inches, which correlate respectively to very coarse, coarse, medium, fine, and very fine granules. Large fragments, or clasts, include all grain sizes larger than 2 mm (5/64 in). These include boulders, cobbles, granules, and gravel. Sand has a grain size between 2 mm and 0.0625 mm, about the lower limit of the naked eye’s resolution. Sediment grains smaller than sand are called silt. Silt is unique; the grains can be felt with a finger or as grit between your teeth, but are too small to see with the naked eye.
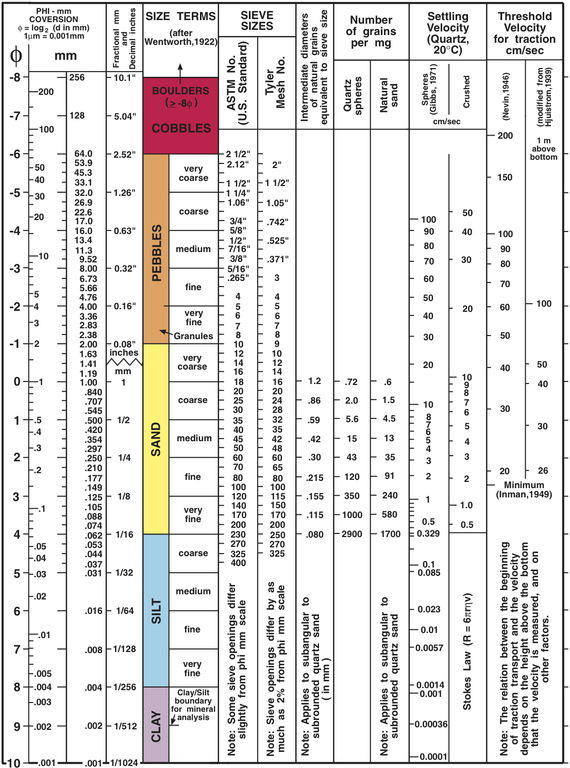
Sorting and Rounding
Sorting describes the range of grain sizes within sediment or sedimentary rock. Geologists use the term “well-sorted” to describe a narrow range of grain sizes, and “poorly sorted” for a wide range of grain sizes (see figure) [11]. It is important to note that soil engineers use similar terms with opposite definitions; well-graded sediment consists of a variety of grain sizes, and poorly graded sediment has roughly the same grain sizes.
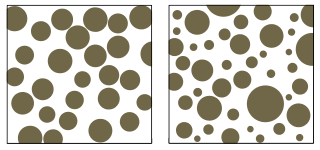
When reading the story told by rocks, geologists use sorting to interpret erosion or transport processes, as well as deposition energy. For example, wind-blown sands are typically extremely well sorted, while glacial deposits are typically poorly sorted. These characteristics help identify the type of erosion process that occurred. Coarse-grained sediment and poorly sorted rocks are usually found nearer to the source of sediment, while fine sediments are carried farther away. In a rapidly flowing mountain stream, you would expect to see boulders and pebbles. In a lake fed by the stream, there should be sand and silt deposits. If you also find large boulders in the lake, this may indicate the involvement of another sediment transport process, such as rockfall caused by ice- or root-wedging.

Rounding is created when angular corners of rock fragments are removed from a piece of sediment due to abrasion during transport. Well-rounded sediment grains are defined as being free of all sharp edges. Very angular sediment retains the sharp corners. Most clast fragments start with some sharp edges due to the bedrock’s crystalline structure, and those points are worn down during transport. More rounded grains imply a longer erosion time or transport distance, or more energetic erosional process. Mineral hardness is also a factor in rounding.
Composition and Provenance
Composition describes the mineral components found in sediment or sedimentary rock and may be influenced by local geology, like source rock and hydrology. Other than clay, most sediment components are easily determined by visual inspection (see Chapter 3, Minerals). The most commonly found sediment mineral is quartz because of its low chemical reactivity and high hardness, making it resistant to weathering, and its ubiquitous occurrence in continental bedrock. Other commonly found sediment grains include feldspar and lithic fragments. Lithic fragments are pieces of fine-grained bedrock [12], and include mud chips, volcanic clasts, or pieces of slate.
Weathering of volcanic rock produces Hawaii’s famous black (basalt) and green (olivine) sand beaches, which are rare elsewhere on Earth. This is because the local rock is composed almost entirely of basalt and provides an abundant source of dark-colored clasts loaded with mafic minerals. According to the Goldich Dissolution Series, clasts high in mafic minerals are more easily destroyed compared to clasts composed of felsic minerals like quartz [13].

Geologists use provenance to discern the original source of sediment or sedimentary rock. Provenance is determined by analyzing the mineral composition and types of fossils present, as well as textural features like sorting and rounding. Provenance is important for describing tectonic history [14], visualizing paleogeographic formations [15], unraveling an area’s geologic history, or reconstructing past supercontinents [16].
In quartz sandstone, sometimes called quartz arenite (SiO2), provenance may be determined using a rare, durable clast mineral called zircon (ZrSiO4). Zircon, or zirconium silicate, contains traces of uranium, which can be used for age-dating the source bedrock that contributed sediment to the lithified sandstone rock (see Chapter 7, Geologic Time).
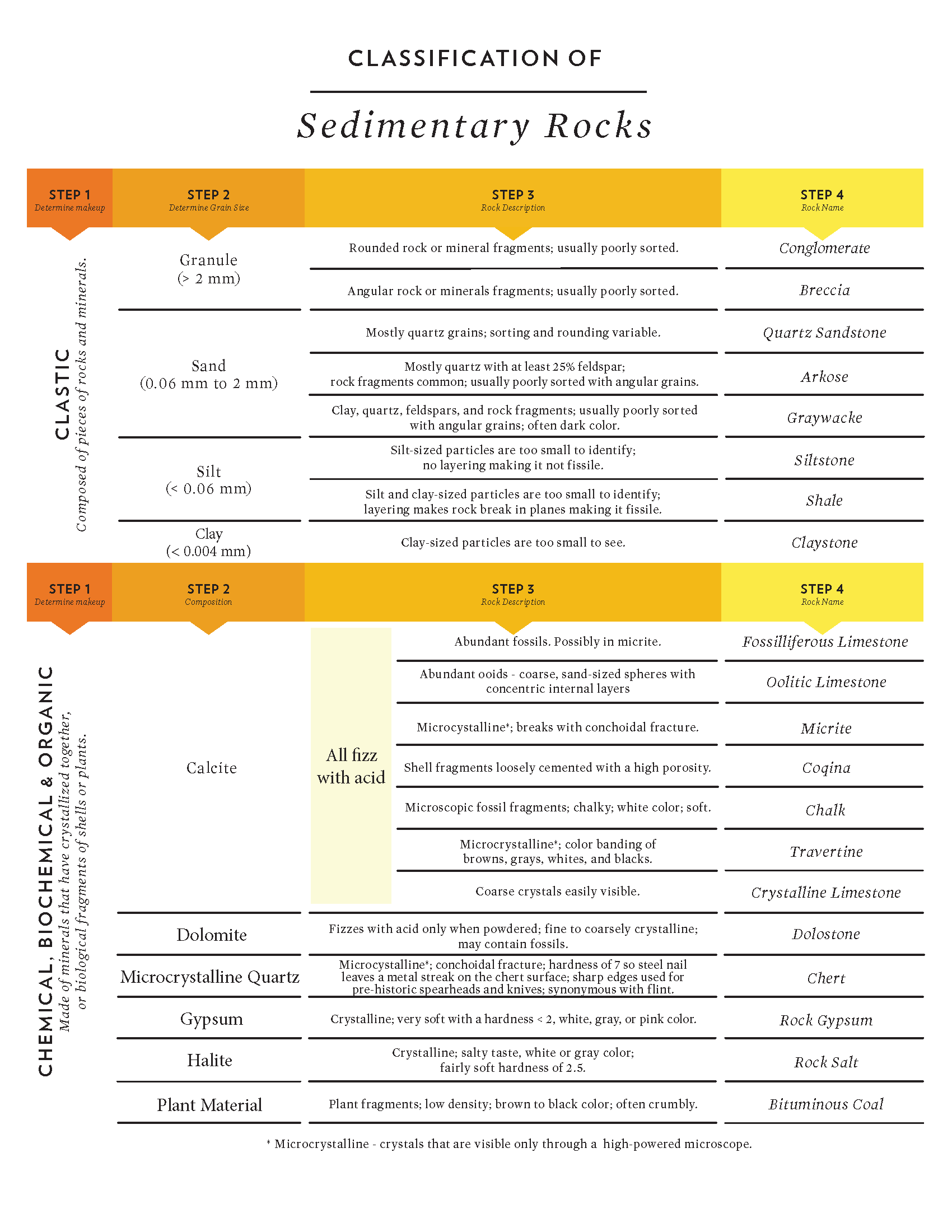
Classification of Clastic Rocks

Clastic rocks are classified according to the grain size of their sediment [17]. Coarse-grained rocks contain clasts with a predominant grain size larger than sand. Typically, smaller sediment grains, collectively called groundmass or matrix, fill in much of the volume between the larger clasts, and hold the clasts together. Conglomerates are rocks containing coarse rounded clasts, and breccias contain angular clasts (see figure). Both conglomerates and breccias are usually poorly sorted.

Medium-grained rocks composed mainly of sand are called sandstone, or sometimes arenite if well sorted. Sediment grains in sandstone can having a wide variety of mineral compositions, roundness, and sorting. Some sandstone names indicate the rock’s mineral composition. Quartz sandstone contains predominantly quartz sediment grains. Arkose is sandstone with significant amounts of feldspar, usually greater than 25%. Sandstone that contains feldspar, which weathers more quickly than quartz, is useful for analyzing the local geologic history. Greywacke is a term with conflicting definitions [18]. Greywacke may refer to sandstone with a muddy matrix, or sandstone with many lithic fragments (small rock pieces).

Fine-grained rocks include mudstone, shale, siltstone, and claystone. Mudstone is a general term for rocks made of sediment grains smaller than sand (less than 2 mm). Rocks that are fissile, meaning they separate into thin sheets, are called shale. Rocks exclusively composed of silt or clay sediment, are called siltstone or claystone, respectively. These last two rock types are rarer than mudstone or shale.

Rock types found as a mixture between the main classifications may be named using the less-common component as a descriptor. For example, a rock containing some silt but mostly rounded sand and gravel are called silty conglomerate. Sand-rich rock containing minor amounts of clay is called clayey sandstone.
Chemical, Biochemical, and Organic
Chemical sedimentary rocks are formed by processes that do not directly involve mechanical weathering and erosion. Chemical weathering may contribute to the dissolved materials in water that ultimately form these rocks. Biochemical and organic sediments are clastic in the sense that they are made from pieces of organic material that are deposited, buried, and lithified; however, they are usually classified as being chemically produced.
Inorganic chemical sedimentary rocks are made of minerals precipitated from ions dissolved in solution, and created without the aid of living organisms. Inorganic chemical sedimentary rocks form in environments where ion concentration, dissolved gasses, temperatures, or pressures are changing, which causes minerals to crystallize.
Biochemical sedimentary rocks are formed from shells and bodies of underwater organisms. The living organisms extract chemical components from the water and use them to build shells and other body parts. The components include aragonite, a mineral similar to and commonly replaced by calcite, and silica.
Organic sedimentary rocks come from organic material that has been deposited and lithified, usually underwater. The source materials are plant and animal remains that are transformed through burial and heat, and end up as coal, oil, and methane (natural gas).
Inorganic Chemical

Inorganic chemical sedimentary rocks are formed when minerals precipitate out of an aqueous solution, usually due to water evaporation. The precipitate minerals form various salts known as evaporites. For example, the Bonneville Salt Flats in Utah flood with winter rains and dry out every summer, leaving behind salts such as gypsum and halite. The deposition order of evaporites deposit is opposite to their solubility order, i.e. as water evaporates and increases the mineral concentration in solution, less soluble minerals precipitate out sooner than the highly soluble minerals. The deposition order and saturation percentages are depicted in the table, bearing in mind the process in nature may vary from laboratory-derived values [19].
| Mineral sequence | Percent Seawater remaining after evaporation |
|---|---|
| Calcite | 50 |
| Gypsum/anhydrite | 20 |
| Halite | 10 |
| Various potassium and magnesium salts | 5 |


Calcium carbonate-saturated water precipitates porous masses of calcite called tufa. Tufa can form near degassing water and in saline lakes. Waterfalls downstream of springs often precipitate tufa as the turbulent water enhances the degassing of carbon dioxide, which makes calcite less soluble and causes it to precipitate. Saline lakes concentrate calcium carbonate from a combination of wave action causing degassing, springs in the lakebed, and evaporation. In salty Mono Lake in California, tufa towers were exposed after water was diverted and lowered the lake levels.

Cave deposits like stalactites and stalagmites are another form of chemical precipitation of calcite, in a form called travertine. Calcite slowly precipitates from water to form the travertine, which often shows banding. This process is similar to the mineral growth on faucets in your home sink or shower that comes from hard (mineral-rich) water. Travertine also forms at hot springs such as Mammoth Hot Spring in Yellowstone National Park.
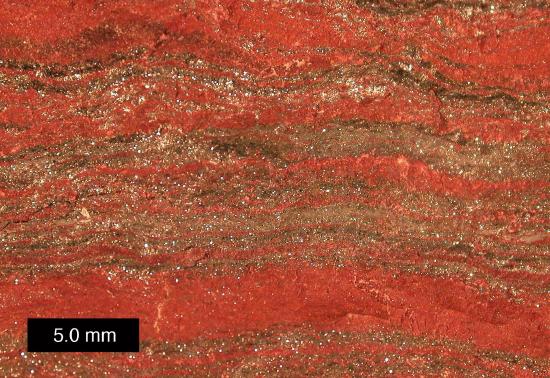
Banded iron formation deposits commonly formed early in Earth’s history, but this type of chemical sedimentary rock is no longer being created. Oxygenation of the atmosphere and oceans caused free iron ions, which are water-soluble, to become oxidized and precipitate out of solution. The iron oxide was deposited, usually in bands alternating with layers of chert.

Chert, another commonly found chemical sedimentary rock, is usually produced from silica (SiO2) precipitated from groundwater. Silica is highly insoluble on the surface of Earth, which is why quartz is so resistant to chemical weathering. Water deep underground is subjected to higher pressures and temperatures, which helps dissolve silica into an aqueous solution. As the groundwater rises toward or emerges at the surface the silica precipitates out, often as a cementing agent or into nodules. For example, the bases of the geysers in Yellowstone National Park are surrounded by silica deposits called geyserite or sinter. The silica is dissolved in water that is thermally heated by a relatively deep magma source. Chert can also form biochemically and is discussed in the Biochemical subsection. Chert has many synonyms, some of which may have gem value such as jasper, flint, onyx, and agate, due to subtle differences in colors, striping, etc., but chert is the more general term used by geologists for the entire group.

Oolites are among the few limestone forms created by an inorganic chemical process, similar to what happens in evaporite deposition. When water is oversaturated with calcite, the mineral precipitates out around a nucleus, a sand grain or shell fragment, and forms little spheres called ooids (see figure). As evaporation continues, the ooids continue building concentric layers of calcite as they roll around in gentle currents.
Biochemical
Biochemical sedimentary rocks are not that different from chemical sedimentary rocks; they are also formed from ions dissolved in solution. However, biochemical sedimentary rocks rely on biological processes to extract the dissolved materials out of the water. Most macroscopic marine organisms use dissolved minerals, primarily aragonite (calcium carbonate), to build hard parts such as shells. When organisms die the hard parts settle as sediment, which becomes buried, compacted, and cemented into rock.

This biochemical extraction and secretion is the main process for forming limestone, the most commonly occurring, non-clastic sedimentary rock. Limestone is mostly made of calcite (CaCO3) and sometimes includes dolomite (CaMgCO3), a close relative. Solid calcite reacts with hydrochloric acid by effervescing or fizzing. Dolomite only reacts to hydrochloric acid when ground into a powder, which can be done by scratching the rock surface (see Chapter 3, Minerals).

Limestone occurs in many forms, most of which originate from biological processes. Entire coral reefs and their ecosystems can be preserved in exquisite detail in limestone rock (see figure). Fossiliferous limestone contains many visible fossils. A type of limestone called coquina originates from beach sands made predominantly of shells that were then lithified. Coquina is composed of loosely-cemented shells and shell fragments. You can find beaches like this in modern tropical environments, such as the Bahamas. Chalk contains high concentrations of shells from a microorganism called a coccolithophore. Micrite, also known as microscopic calcite mud, is a very fine-grained limestone containing microfossils that can only be seen using a microscope.
Biogenetic chert forms on the deep ocean floor, created from biochemical sediment made of microscopic organic shells. This sediment, called ooze, may be calcareous (calcium carbonate-based) or siliceous (silica-based) depending on the type of shells deposited. For example, the shells of radiolarians (zooplankton) and diatoms (phytoplankton) are made of silica, so they produce siliceous ooze.
Organic
Under the right conditions, intact pieces of organic material or material derived from organic sources are preserved in the geologic record. Although not derived from sediment, this lithified organic material is associated with sedimentary strata and created by similar processes—burial, compaction, and diagenesis. Deposits of these fuels develop in areas where organic material collects in large quantities. Lush swamplands can create conditions conducive to the coal formation. Shallow-water, organic material-rich marine sediment can become highly productive petroleum and natural gas deposits. See Chapter 16, Energy and Mineral Resources, for a more in-depth look at these fossil-derived energy sources.

Classification of Chemical Sedimentary Rocks

In contrast to detrital sediment, chemical, biochemical, and organic sedimentary rocks are classified based on mineral composition. Most of these are monomineralic, composed of a single mineral, so the rock name is usually associated with the identifying mineral. Chemical sedimentary rocks consisting of halite are called rock salt. Rocks made of Limestone (calcite) is an exception, having elaborate subclassifications and even two competing classification methods: Folk Classification and Dunham Classification [11; 21]. The Folk Classification deals with rock grains and usually requires a specialized, petrographic microscope. The Dunham Classification is based on rock texture, which is visible to the naked eye or using a hand lens and is easier for field applications. Most carbonate geologists use the Dunham system.
References
11. Folk, R. L. Petrography of sedimentary rocks. Univ. Texas, Hemphill, Austin, Tex 182, (1974).
17. Grabau, A. W. On the classification of sedimentary rocks. (1904).
20. Boggs, S. J. Principles of sedimentology and stratigraphy. (Pearson, 2011).
21. Dunham, R. J. Classification of carbonate rocks according to depositional textures. (1962).


