5.2: Weathering and Erosion
- Page ID
- 6868
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Bedrock refers to the solid crystalline rock that makes up the Earth’s outer crust. Weathering is a process that turns bedrock into smaller particles, called sediment or soil. Mechanical weathering includes pressure expansion, frost wedging, root wedging, and salt expansion. Chemical weathering includes carbonic acid and hydrolysis, dissolution, and oxidation.
Erosion is a mechanical process, usually driven by water, wind, gravity, or ice, which transports sediment and soil from the place of weathering. Liquid water is the main agent of erosion. Gravity and mass wasting processes (see Chapter 10, Mass Wasting) move rocks and sediment to new locations. Gravity and ice, in the form of glaciers (see Chapter 14, Glaciers), move large rock fragments as well as fine sediment.
Erosion resistance is important in the creation of distinctive geological features. This is well-demonstrated in the cliffs of the Grand Canyon. The cliffs are made of rock left standing after less resistant materials have weathered and eroded away. Rocks with different levels of erosion resistance also create unique-looking features called hoodoos in Bryce Canyon National Park and Goblin Valley State Park in Utah.
Mechanical Weathering
Mechanical weathering physically breaks bedrock into smaller pieces. The usual agents of mechanical weathering are pressure, temperature, freezing/thawing cycle of water, plant or animal activity, and salt evaporation.
Pressure Expansion

Bedrock buried deep within the Earth is under high pressure and temperature. When uplift and erosion brings bedrock to the surface, its temperature drops slowly, while its pressure drops immediately. The sudden pressure drop causes the rock to rapidly expand and crack; this is called pressure expansion. Sheeting or exfoliation is when the rock surface spalls off in layers. Spheroidal weathering is a type of exfoliation that produces rounded features and is caused when chemical weathering moves along joints in the bedrock.
Frost Wedging
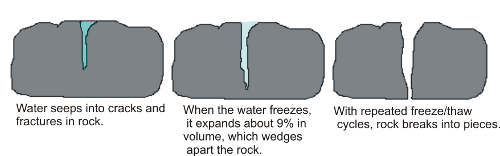
Frost wedging, also called ice wedging, uses the power of expanding ice to break apart rocks. Water works its way into various cracks, voids, and crevices. As the water freezes, it expands with great force, exploiting any weaknesses. When ice melts, the liquid water moves further into the widened spaces. Repeated cycles of freezing and melting eventually pry the rocks apart. The cycles can occur daily when fluctuations of temperature between day and night go from freezing to melting.
Root Wedging

Like frost wedging, root wedging happens when plant roots work themselves into cracks, prying the bedrock apart as they grow. Occasionally these roots may become fossilized. Rhizolith is the term for these roots preserved in the rock record [2]. Tunneling organisms such as earthworms, termites, and ants are biological agents that induce weathering similar to root wedging.
Salt Expansion

Salt expansion, which works similarly to frost wedging, occurs in areas of high evaporation or near-marine environments. Evaporation causes salts to precipitate out of solution and grow and expand into cracks in the rock. Salt expansion is one of the causes of tafoni, a series of holes in a rock. Tafoni, cracks, and holes are weak points that become susceptible to increased weathering. Hopper crystal describes a square-shaped crystal, commonly made of salt, preserved in rock.
Chemical Weathering
Chemical weathering is the dominant weathering process in warm, humid environments. It happens when water, oxygen, and other reactants chemically degrade the mineral components of bedrock and turn them into water-soluble ions which can then be transported by water. Higher temperatures accelerate chemical weathering rates.
Chemical and mechanical weathering work hand-in-hand via a fundamental concept called surface-area-to-volume ratio. Chemical weathering only occurs on rock surfaces because water and reactants cannot penetrate the solid rock. Mechanical weathering penetrates the bedrock, breaking large rocks into smaller pieces and creating new rock surfaces. This exposes more surface area to chemical weathering, enhancing its effects. In other words, higher surface-area-to-volume ratios produce higher rates of overall weathering.
Carbonic Acid and Hydrolysis
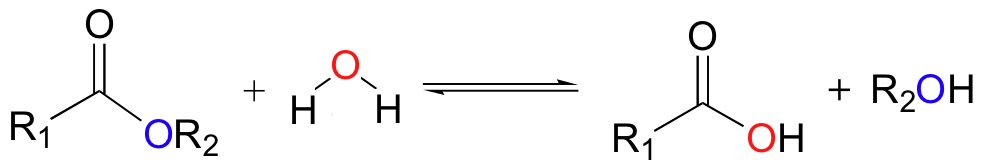
Carbonic acid (H2CO3) forms when carbon dioxide, the fifth-most abundant gas in the atmosphere, dissolves in water. This happens naturally in clouds, which is why precipitation is normally slightly acidic. Carbonic acid is an important agent in two chemical weathering reactions, hydrolysis, and dissolution.
Hydrolysis occurs via two types of reactions. In one reaction, water molecules ionize into positively charged H+ and OH− ions and replace mineral cations in the crystal lattice. In another type of hydrolysis, carbonic acid molecules react directly with minerals, especially those containing silicon and aluminum (i.e. Feldspars), to form molecules of clay minerals.
Hydrolysis is the main process that breaks down silicate rock and creates clay minerals. The following is a hydrolysis reaction that occurs when silica-rich feldspar encounters carbonic acid to produce water-soluble clay and other ions:
feldspar + carbonic acid (in water) → clay + metal cations (Fe++, Mg++, Ca++, Na+, etc.) + bicarbonate anions (HCO3-) + silica (SiO2)
Clay minerals are platy silicates or phyllosilicates (see Chapter 3, Minerals) similar to micas, and are the main components of very fine-grained sediment. The dissolved substances may later precipitate into chemical sedimentary rocks like evaporite and limestone, as well as amorphous silica or chert nodules.
Dissolution

Dissolution is a hydrolysis reaction that dissolves minerals in bedrock and leaves the ions in solution, usually in water. Some evaporites and carbonates, like salt and calcite, are more prone to this reaction; however, all minerals can be dissolved. Non-acidic water, having a neutral pH of 7, will dissolve any mineral, although it may happen very slowly. Water with higher levels of acid, natural or man-made, dissolves rocks at a higher rate. Liquid water is normally slightly acidic due to the presence of carbonic acid and free H+ ions. Natural rainwater can be highly acidic, with pH levels as low as 2 [3]. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. Regions with high humidity (airborne moisture) and precipitation experience more dissolution due to greater contact time between rocks and water.

The Goldich Dissolution Series shows chemical weathering rates are associated with crystallization rankings in the Bowen’s Reaction Series (see Chapter 4, Igneous Rock and Volcanic Processes) [4]. Minerals at the top of the Bowen series crystallize under high temperatures and pressures, and chemically weather at a faster rate than minerals ranked at the bottom. Quartz, a felsic mineral that crystallizes at 700°C, is very resistant to chemical weathering. High crystallization-point mafic minerals, such as olivine and pyroxene (1,250°C), weather relatively rapidly and more completely. Olivine and pyroxene are rarely found as end products of weathering because they tend to break down into elemental ions.


Dissolution is also noteworthy for the special geological features it creates. In places with abundant carbonate bedrock, dissolution weathering can produce a karst topography characterized by sinkholes or caves (see Chapter 10, Mass Wasting).
Timpanogos Cave National Monument in Northern Utah is a well-known dissolution feature. The figure shows a cave formation created from dissolution followed by precipitation—groundwater saturated with calcite seeped into the cavern, where evaporation caused the dissolved minerals to precipitate out.
Oxidation

Oxidation, the chemical reaction that causes rust in metallic iron, occurs geologically when iron atoms in a mineral bond with oxygen. Any minerals containing iron can be oxidized. The resultant iron oxides may permeate a rock if it is rich in iron minerals. Oxides may also form a coating that covers rocks and grains of sediment, or lines rock cavities and fractures. If the oxides are more susceptible to weathering than the original bedrock, they may create void spaces inside the rock mass or hollows on exposed surfaces.
Three commonly found minerals are produced by iron-oxidation reactions: red or grey hematite, brown goethite (pronounced “GUR-tite”), and yellow limonite. These iron oxides coat and bind mineral grains together into sedimentary rocks in a process called cementation and often give these rocks a dominant color. They color the rock layers of the Colorado Plateau, as well as Zion, Arches, and Grand Canyon National Parks. These oxides can permeate a rock that is rich in iron-bearing minerals or can be a coating that forms in cavities or fractures. When the minerals replacing existing minerals in bedrock are resistant to weathering, iron concretions may occur in the rock. When bedrock is replaced by weaker oxides, this process commonly results in void spaces and weakness throughout the rock mass and often leaves hollows on exposed rock surfaces.
Erosion

Erosion is a mechanical process, usually driven by water, gravity, (see Chapter 10), wind, or ice (see Chapter 14) that removes sediment from the place of weathering. Liquid water is the main agent of erosion.

Erosion resistance is important in the creation of distinctive geological features. This is well demonstrated in the cliffs of the Grand Canyon. The cliffs are made of rock left standing after less resistant materials have weathered and eroded away. Rocks with different levels of erosion resistant also create a unique-looking features called hoodoos in Bryce Canyon National Park and Goblin Valley State Park in Utah.
Soil
Soil is a combination of air, water, minerals, and organic matter that forms at the transition between the biosphere and geosphere. Soil is made when weathering breaks down the bedrock and turns it into sediment. If erosion does not remove the sediment significantly, organisms can access the mineral content of the sediments. These organisms turn minerals, water, and atmospheric gases into organic substances that contribute to the soil.
Soil is an important reservoir for organic components necessary for plants, animals, and microorganisms to live. The organic component of soil, called humus, is a rich source of bioavailable nitrogen. Nitrogen is the most common element in the atmosphere, but it exists in a form most life forms are unable to use. Special bacteria found only in the soil provide most nitrogen compounds that are usable, bioavailable, by life forms.
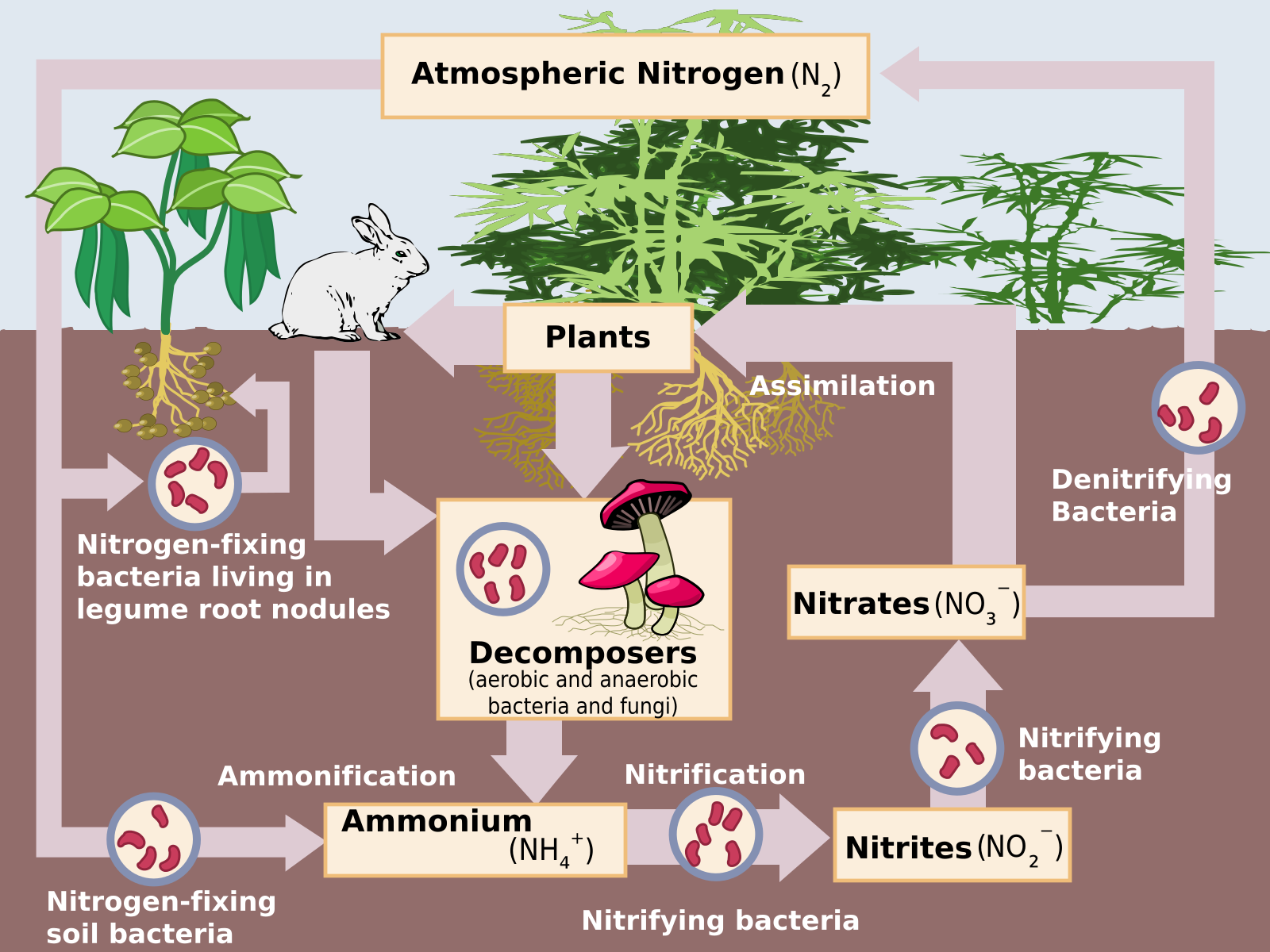
These nitrogen-fixing bacteria absorb nitrogen from the atmosphere and convert it into nitrogen compounds. These compounds are absorbed by plants and used to make DNA, amino acids, and enzymes. Animals obtain bioavailable nitrogen by eating plants, and this is the source of most of the nitrogen used by life. That nitrogen is an essential component of proteins and DNA. Soils range from poor to rich, depending on the amount of humus they contain. Soil productivity is determined by water and nutrient content. Freshly created volcanic soils, called andisols, and clay-rich soils that hold nutrients and water are examples of productive soils.

The nature of the soil, meaning its characteristics, is determined primarily by five components:
- The mineralogy of the parent material
- Topography
- Weathering
- Climate
- The organisms that inhabit the soil.
For example, soil tends to erode more rapidly on steep slopes so soil layers in these areas may be thinner than in flood plains, where it tends to accumulate. The quantity and chemistry of organic matter of soil affect how much and what varieties of life it can sustain. Temperature and precipitation, two major weathering agents, are dependent on climate. Fungi and bacteria contribute organic matter and the ability of soil to sustain life, interacting with plant roots to exchange nitrogen and other nutrients [5].
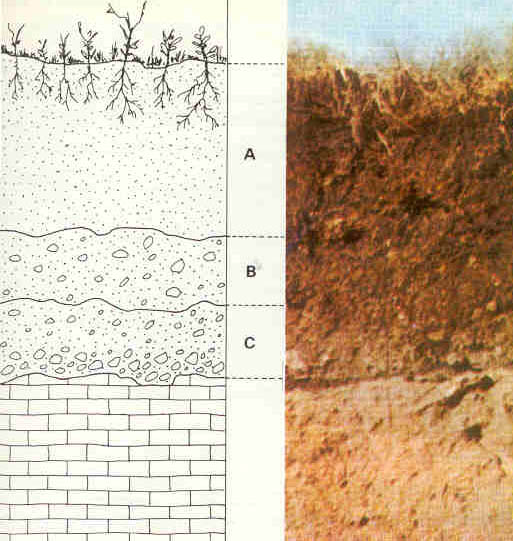
In well-formed soils, there is a discernable arrangement of distinct layers called soil horizons [6]. These soil horizons can be seen in road cuts that expose the layers at the edge of the cut. Soil horizons make up the soil profile. Each soil horizon reflects climate, topography, and other soil-development factors, as well as its organic material and mineral sediment composition. The horizons are assigned names and letters. Differences in naming schemes depend on the area, soil type or research topic. The figure shows a simplified soil profile that uses commonly designated names and letters.
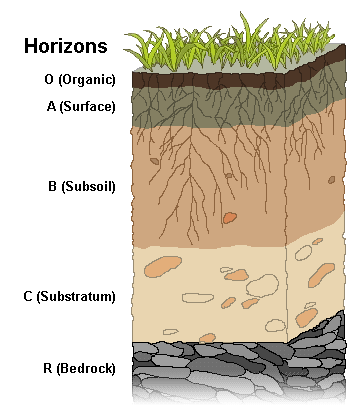
O Horizon: The top horizon is a thin layer of predominantly organic material, such as leaves, twigs, and other plant parts that are actively decaying into humus.
A Horizon: The next layer, called topsoil, consists of humus mixed with mineral sediment. As precipitation soaks down through this layer, it leaches out soluble chemicals. In wet climates with heavy precipitation, this leaching out produces a separate layer called horizon E, the leaching or eluviation zone.
B Horizon: Also called subsoil, this layer consists of sediment mixed with humus removed from the upper layers. The subsoil is where mineral sediment is chemically weathered. The amount of organic material and the degree of weathering decrease with depth. The upper subsoil zone, called regolith, is a porous mixture of humus and highly weathered sediment. In the lower zone, saprolite, scant organic material is mixed with largely unaltered parent rock.
C Horizon: This is substratum and is a zone of mechanical weathering. Here, bedrock fragments are physically broken but not chemically altered. This layer contains no organic material.
R Horizon: The final layer consists of unweathered, parent bedrock and fragments.

The United States governing body for agriculture, the USDA, uses a taxonomic classification to identify soil types, called soil orders. Xoxisols or laterite soils are nutrient-poor soils found in tropical regions. While poorly suited for growing crops, xosisols are home to most of the world’s mineable aluminum ore (bauxite). Ardisol forms in dry climates and can develop layers of hardened calcite, called caliche. Andisols originate from volcanic ash deposits. Alfisols contain silicate clay minerals. These two soil orders are productive for farming due to their high content of mineral nutrients. In general, color can be an important factor in understanding soil conditions. Black soils tend to be anoxic, red oxygen-rich, and green oxygen-poor (i.e. reduced). This is true for many sedimentary rocks as well.

Not only is soil essential to terrestrial life in nature, but also human civilization via agriculture. Careless or uninformed human activity can seriously damage soil’s life-supporting properties. A prime example is the famous Dust Bowl disaster of the 1930s, which affected the midwestern United States. The damage occurred because of large-scale attempts to develop prairie land in southern Kansas, Colorado, western Texas, and Oklahoma into farmland [7]. Poor understanding of the region’s geology, ecology, and climate led to farming practices that ruined the soil profile.
The prairie soils and native plants are well adapted to a relatively dry climate. With government encouragement, settlers moved in to homestead the region. They plowed vast areas of prairie into long, straight rows and planted grain. The plowing broke up the stable soil profile and destroyed the natural grasses and plants, which had long roots that anchored the soil layers. The grains they planted had shallower root systems and were plowed up every year, which made the soil prone to erosion. The plowed furrows were aligned in straight rows running downhill, which favored erosion and loss of topsoil.
The local climate does not produce sufficient precipitation to support non-native grain crops, so the farmers drilled wells and over-pumped water from the underground aquifers. The grain crops failed due to lack of water, leaving bare soil that was stripped from the ground by the prairie winds. Particles of midwestern prairie soil were deposited along the east coast and as far away as Europe. Huge dust storms called black blizzards made life unbearable, and the once-hopeful homesteaders left in droves. The setting for John Steinbeck’s famous novel and John Ford’s film, The Grapes of Wrath, is Oklahoma during this time. The lingering question is whether we have learned the lessons of the dust bowl, to avoid creating it again [8].


