1.12: Lab 12 - Introduction to Minerals and Rocks
- Page ID
- 25336
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This lab contains potentially inaccessible interactive resources. Please work with your instructor and local campus resources to identify accommodations for these resources.
- Describe the basic mineral properties and identify common rock-forming minerals.
- Explain the rock cycle and the processes that form igneous, sedimentary, and metamorphic rocks.
- Distinguish among igneous, sedimentary, and metamorphic rock samples.
Introduction
The minerals identified in this lab represent a small collection of the most common rock-forming minerals. To understand the relationship between minerals and rocks, think of a candy bar made up of several different materials such as chocolate, sugar, nuts, and caramel. The minerals are the ingredients (chocolate, sugar, nuts, caramel) that make up a rock (the candy bar). Minerals differ from each other in chemical composition and structure. Rocks differ from each other based on the environmental setting in which they formed. This lab will introduce you to the three families of rocks as well as the environment and material needed to create them. You will learn how these rocks interact in what is known as the rock cycle. And, you will learn the basics in mineral and rock identification by utilizing your observation and interpretation skills.
Part A. Introduction to Minerals
A mineral must satisfy all four of the following criteria:
- Minerals are a naturally occurring substance (not made by humans),
- Minerals are an inorganic substance (not composed of living or once living organisms),
- Minerals have a fixed chemical composition (elements), and
- Minerals have an orderly internal structure (based on how the elements bond with each other).
 Check It Out! How Many Minerals Are There?
Check It Out! How Many Minerals Are There?
There are approximately 4,000 different minerals found on Earth, and each of those minerals have a unique set of properties. Some minerals are abundant, like quartz, while others are rare, like the California State gemstone benitoite. Check out the California Department of Conservation website to learn more about the State gemstone!
The vast majority of the minerals that make up the rocks of Earth’s crust are silicate minerals. These include minerals such as quartz, feldspar, mica, amphibole, pyroxene, olivine, and a great variety of clay minerals. Lighter-colored silicates are called felsic, as they lack iron and magnesium, while darker-colored minerals are called mafic, as they do contain iron and magnesium. The building block of all of these minerals is the silica tetrahedron, a combination of four oxygen atoms and one silicon atom.[205] The remaining minerals are non-silicate minerals, meaning that they do not have silicon as part of their chemistry. Non-silicates include sulfides, oxides, native elements, sulfates, carbonates, and halides. Often these minerals are formed in either sedimentary or metamorphic rocks.
 Check It Out! Silicate vs. Non-Silicate Minerals
Check It Out! Silicate vs. Non-Silicate Minerals
Watch a tutorial video on the differences between silicate and non-silicate minerals, and learn how to identify the differences between these two types of minerals. (Video length is 4:25).
Conflict Minerals
Some mineral mining operations fund violence and cause human rights abuses. You may have heard of “blood diamonds” or “dirty gold” before. Conflict minerals are mined in locations with political instability, corrupt governments, armed conflict, and/or lax regulations and then sold on the global market; profits are used to perpetuate further violence and human rights abuses. By increasing the funds of armed groups, mine profits increase fighting and spread violence. Conflict mineral violence has been documented in numerous countries including Colombia, Myanmar (Burma), Côte d'Ivoire, and other countries. A 2012 U.S. federal law requires companies to publicly disclose their use of the conflict minerals that are the source of tantalum, tin, gold, and tungsten that originate in the Democratic Republic of the Congo or a neighboring country. These four minerals are used in electronic products like cell phones and laptops. Conflict minerals compose end-user products through a complex chain of local and international traders, processors, and manufacturers.
Let’s take a closer look at the minerals that provide the metals identified in the 2012 law.
➢ The metal tantalum (symbol Ta) is found in the mineral tantalite. The chemical formula for tantalite is (Fe, Mn)Ta2O6—either one iron atom or one manganese atom bonds with two tantalum atoms, and six oxygen atoms. Tantalum is used in electronics as a capacitor.
➢ The metal tin (symbol Sn) is found in the mineral cassiterite. The chemical formula for cassiterite is SnO2—one tin atom is bonded to two oxygen atoms. Tin is used as a sorter on circuit boards in electronics.
➢ The metal gold (symbol Au) is often found in its elemental form as gold nuggets, as veins in rocks, or in alluvial (river) deposits. Gold is used as a coating for electric wires in electronic devices.
➢ The metal tungsten (W) is found in the mineral wolframite. The chemical formula for wolframite is (Fe,MN)WO4—either one iron or one manganese atom bonds with one tungsten atom and four oxygen atoms. Tungsten is used for electronic filaments and gives mobile phones their vibrating capacity.
- Select either tantalite, cassiterite, or wolframite and explain how this mineral meets the four criteria of a mineral. Also indicate if the mineral is a silicate or non-silicate mineral.
Basic Mineral Properties
Minerals can be identified by their physical characteristics. The physical properties of minerals are related to their chemical composition and bonding. Some characteristics, such as a mineral’s hardness, are more useful for mineral identification. Color is readily observable and certainly obvious, but it is usually less reliable than other physical properties.[206] The five basic properties you will investigate for mineral identification are as follows: color, streak, luster, hardness, and cleavage/fracture.
- Using the photographed specimen below (Figure 12.1), answer the following questions:
- What is the general color of the specimen?
- What is the general shape (square, triangle, etc.) of the specimen?
- Is the specimen shiny like glass, reflective like metal, or greasy like a candle?
- If the specimen were dropped, do you think it would shatter or break into a geometric design?

 Think About It…Why Is This Important?
Think About It…Why Is This Important?
The previous question provided you the general steps on identifying a mineral. It is important to know that when asking critical and constructive questions it is best not to make a judgment after the first questions. Following a set of rules or guidelines ensures the best results. So, don’t jump to conclusions as you follow the instructions on how to identify minerals and rocks in the upcoming exercises.
Color
For most of us, color is one of our key ways of identifying objects. While some minerals have particularly distinctive colors that make good diagnostic properties, many do not. For many others, color is simply unreliable. The mineral sulphur is always a distinctive and unique yellow. Hematite, on the other hand, is an example of a mineral for which color is not diagnostic. In some forms hematite is deep dull red, but in others it is black and shiny metallic. Many other minerals can have a wide range of colors (e.g., quartz, feldspar, amphibole, fluorite, and calcite). In most cases, the variations in colors are a result of varying proportions of trace elements within the mineral. In the case of quartz, for example, yellow quartz (citrine) has trace amounts of ferric iron (Fe3+), rose quartz has trace amounts of manganese, purple quartz (amethyst) has trace amounts of iron, and milky quartz, which is very common, has millions of fluid inclusions (tiny cavities, each filled with water).[208]
Search the internet or use the QR codes/website links shown below to research mineral colors.
 Identify four different variations or types of quartz, as well as their general color.
Identify four different variations or types of quartz, as well as their general color.
- Variation 1 (name and color):
- Variation 2 (name and color):
- Variation 3 (name and color):
- Variation 4 (name and color):
 Identify four different minerals that can be green.
Identify four different minerals that can be green.
- Mineral 1:
- Mineral 2:
- Mineral 3:
- Mineral 4:
 Identify four different minerals that can be blue.
Identify four different minerals that can be blue.
- Mineral 1:
- Mineral 2:
- Mineral 3:
- Mineral 4:
- Explain why although the most obvious tool for mineral identification is the color, it is not the most accurate or definitive tool. Your response should be one to two sentences in length.
 Check It Out! Minerals in Fireworks!?
Check It Out! Minerals in Fireworks!?
Minerals provide the colors for fireworks. Check out this link by the United States Geologic Survey that identifies which minerals produce some of your favorite colors in fireworks!
Streak
Streak is the color of a mineral’s powder. Streak is a more reliable property than color because streak does not vary. Minerals that are the same color may have a different colored streak.[209] Many minerals, such as quartz, do not have a streak, as they are harder than the streak plate. To check the streak, you must scrape the mineral across an unglazed white porcelain plate.
- Observe the streak provided below (Figure 12.2). The photograph shows that the streak color is similar although the specimen colors are not. These two samples are the same mineral, which is why the streak plate color is the same, so why do you think the samples look different? Tip: read the Think About It box below.

 Think About It…How Can These Samples Be the Same Mineral?
Think About It…How Can These Samples Be the Same Mineral?
Hematite is a common iron oxide mineral, which means that this mineral can rust. The name hematite is derived from the Greek word haima, meaning blood, which makes reference to the mineral’s streak! Fun fact: hematite is used as a pigment in red paint and is used in some makeups as a natural glitter!
Luster
Luster is the way light reflects off the surface of a mineral, and the degree to which it penetrates into the interior. The key distinction is between metallic and non-metallic lusters. Light does not pass through metals, and that is the main reason they look “metallic”. Even a thin sheet of metal—such as aluminum foil—will prevent light from passing through it. Many non-metallic minerals may look as if light will not pass through them, but if you take a closer look at a thin edge of the mineral you can see that it does. If a non-metallic mineral has a shiny, reflective surface, then it is called glassy. If it is dull and non-reflective, it is called earthy. Other types of non-metallic lusters are waxy (like a crayon or candle) or pearly (like a shell). Luster is a good diagnostic property, since most minerals will always appear either metallic or non-metallic.[211]
Use the luster flow chart (Figure 12.3) to answer the following questions about the two specimens pictured in Figure 12.4.
- Sample A (Figure 12.4, left photograph):
- What is the luster?
- What is the color?
- Sample B (Figure 12.4, right photograph):
- What is the luster?
- What is the color?
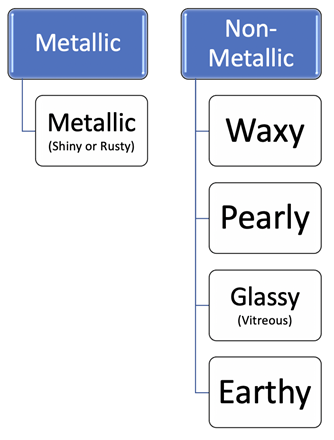

 Check It Out! Mineral Luster
Check It Out! Mineral Luster
This lab simplifies luster to five options, but the Mineralogical Society of America acknowledges 10 different types of luster!
Hardness
Mohs' scale of mineral hardness is named after Sir Friedrich Mohs, a mineralogist who invented a scale of hardness based on the ability of one mineral to scratch another. According to the scale, talc is the softest: it can be scratched by all other materials. Gypsum is harder as it can scratch talc but not calcite, which is even harder. An easy benchmark to remember is that the human fingernail has a hardness of about 2.5, so if you can scratch it with your nail, it must be softer than a 2.5. The hardness of a mineral is controlled mainly by the strength of the bonding between the atoms and partly by the size of the atoms. It is a measure of the resistance of the mineral to scratching. Mohs’ scale is for natural minerals. Diamond is always at the top of Mohs’ scale, being the hardest mineral. There are ten minerals in Mohs’ scale (from softest to hardest): talc, gypsum, calcite, fluorite, apatite, feldspar, quartz, topaz, corundum, and diamond (Figure 12.5).[213]

Cleavage and Fracture
The way in which a mineral breaks is determined by the arrangement of its atoms and the chemical bond between those atoms. It is difficult for us to observe these bonds, but we can observe the shape and design into which minerals break. A mineral that exhibits cleavage will consistently break, or cleave, along a parallel flat surface. Muscovite mica is a good example as it breaks along very closely spaced flat planes that yield thin sheets. We would identify this as having one plane of cleavage (a plane represents a flat surface with a top and bottom). If a mineral had six flat sides, we would say that the specimen has three planes of cleavage. A helpful way of visualizing cleavage is with the use of an ax (Figure 12.6). An ax makes a single cut, defining a top and bottom, so, if a specimen needs two axes, it would have two directions of cleavage, and have four flat sides.

Some minerals will be observed in a solid crystalline form. The arrangement of the atoms within the mineral will determine the external shapes, and as the mineral begins to solidify, these microscopic crystals, or seeds, will form and begin to grow. The longer the mineral has to form, the larger the crystal. Other minerals fracture, which means that they break in a rough or irregular pattern. Some minerals, such as quartz, will fracture along the surface and create a conchoidal fracture. A conchoidal fracture does not have any surface planes like those seen in Figure 12.6.
 Think About It…California Beach Sand?
Think About It…California Beach Sand?
Most of the sand that is found along California’s beaches are composed of quartz and feldspar crystals. Quartz is known for its conchoidal fracture. Conchoidal fractures result in a curved breakage, that resembles the gradual curves of a mussel shell, which is the origin of the name! Next time you are at the beach, examine a quartz grain up close to see its conchoidal fracture.
Now that you understand mineral cleavage and fracture, answer the following questions about the two samples shown in Figure 12.7.
- Sample A (Figure 12.7, left shape):
- If you had to describe this sample as a shape, what shape would it be?
- How many sides does this sample have?
- How many planes or directions of cleavage does this sample have?
- Sample B (Figure 12.7, right shape):
- If you had to describe this sample as a shape, what shape would it be?
- How many sides does this sample have?
- How many planes or directions of cleavage does this sample have?

Part B. Mineral Identification
 Guided Practice: Mineral Identification
Guided Practice: Mineral Identification
Go to this tutorial video from Professor Jeremy Patrich on how to identify minerals based on their properties. (Video length is 7:27).
How to Use the Basic Mineral Identification Table
As you have learned up to this point, minerals have many physical properties. Table 12.1 provides different properties of minerals organized by hardness (the softest minerals are at the top and the hardest minerals are at the bottom). Remember that your fingernail has a hardness of 2.5. If a specimen has most or all of the properties along a horizontal row, then the name of that sample is shown on the far-left column.
| Mineral Name | Mineral Hardness | Mineral Color | Mineral Luster | Mineral Streak | Cleavage or Fracture | Other Properties |
|---|---|---|---|---|---|---|
| Talc | 1 | White to Gray White | Waxy to Pearly | White | Cleavage: 1 Direction | Feels Slippery |
| Gypsum | 2 | Colorless to Brownish | Pearly | White | Cleavage: 1 Direction | Used in Drywall |
| Muscovite Mica | 2-2.5 | White to Greenish | Vitreous (Glassy) | White | Cleavage: 1 Direction | Is Elastic, Peels into Sheets |
| Galena | 2.5 | Light to Dark Gray | Metallic | Grayish- Black | Forms in Perfect Cubes | Is a Lead Sulfide (Very Dense) |
| Halite | 2.5 | White to Pink | Vitreous | White | Cleavage: 3 Directions (Square) | Salty in Taste |
| Biotite Mica | 2.5-3 | Dark Brown to Blackish | Vitreous to Pearly | Gray | Cleavage: 1 Direction | Is Elastic, Peels into Sheets |
| Calcite | 3 | Clear, Blue, Pink, Green, to Brown | Vitreous to Earthy | White | Cleavage: 3 Directions (Rhombohedral) | Double Refraction |
| Azurite | 3.4-4 | Blue | Vitreous to Earthy | Light Blue | Conchoidal Fracture | Mammillary |
| Malachite | 3.6-4 | Green | Vitreous to Waxy | Light Green | Fracture is Uneven: Breaks | Copper Ore |
| Flourite | 4 | White, Green, Yellow, to Red | Vitreous | White | Cleavage: 4 Octahedral (8 sides) | Glows When Exposed to UV Light |
| Kyanite | 4-6.5 | Blue to Black | Vitreous or Pearly | White | Elongated or Bladed Crystals | Fibrous and Flaky |
| Apatite | 5 | Lime Green to Brown | Vitreous to Resinous | White | Hexagonal Prisms | Glows When Exposed to UV Light |
| Hematite | 5.5-6 | Reddish to Blackish | Metallic | Reddish- Brown | Flaky | Used as Glitter (unless oxidized) |
| Orthoclase Feldspar | 6 | White to Pink | Vitreous | White | Cleavage: 2 Blocky | Looks Like a Broken Brick |
| Pyrite | 6.5 | Gold | Metallic | Greenish- Black | Forms in Perfect Cubes | Is a Lead Sulfide (Very Dense) |
| Olivine | 6.5 | Yellow Green to Green | Vitreous | White | Conchoidal Fracture | Crystals Will Have 6 Sides |
| Quartz | 7 | Clear to Brown | Vitreous | White | Conchoidal Fracture | Crystals Will Have 6 Sides |
| Topaz | 8 | Clear to Red | Vitreous | White | Conchoidal Fracture | Used as a Gemstone |
| Corundum | 9 | Red to Gray | Vitreous | None | None- Crystal is Hexagonal | Extremely Hard |
Mineral Identification Activity (Hands-On Version)
Using the tools provided and Table 12.1, identify the mineral samples provided by your instructor. Write your answers in question 12 below.
Mineral Identification Activity (Digital Version)
Your instructor will indicate which playlist (set 1, 2, or 3) you should use for this activity. Each playlist begins with the Guided Practice video on mineral identification followed by six mineral specimens. Click on the correct QR code or link to access the playlist. Be sure to read the mineral properties shown in the video details and use Table 12.1 to identify each specimen. Write your answers in question 12 below. Note: the mineral specimen videos provided in the playlists do not have audio.
Playlist 1:  Playlist 2:
Playlist 2:  Playlist 3:
Playlist 3: 
Mineral Identification Activity: Your Answers
- Identify the minerals.
- Sample __________ (number or letter) is the mineral: _______________________
- Sample __________ (number or letter) is the mineral: _______________________
- Sample __________ (number or letter) is the mineral: _______________________
- Sample __________ (number or letter) is the mineral: _______________________
- Sample __________ (number or letter) is the mineral: _______________________
- Sample __________ (number or letter) is the mineral: _______________________
Part C. Rocks and Their Properties
Rocks are naturally occurring aggregates of one or more minerals. The rock components of the crust are slowly but constantly being changed from one form to another and the processes involved are summarized in the rock cycle (Figure 12.8). The rock cycle is driven by two forces:
- Earth’s internal heat engine, which moves material around in the core and the mantle and leads to slow but significant changes within the crust, and
- The hydrological cycle, which is the movement of liquid water, ice, and water vapor at or near the surface of the Earth, and is powered by the Sun.[217]
The rock cycle is still active on Earth because our core is hot enough to keep the mantle convecting, our atmosphere is relatively thick, and we have liquid water. On some other planets or their satellites, such as the Moon, the rock cycle is virtually dead because the core is no longer hot enough to drive mantle convection and there is no atmosphere or liquid water.8

- Refer to Figure 12.8.
- What types of rock form from the cooling of magma?
- What are the four processes that create sediments?
- What rock is formed when sediments are buried, compacted, and cemented?
- What three arrows are pointing to the metamorphic rock box? Hint: these are the three rock types that can be transformed into a metamorphic rock through burial, heat, and pressure.
Igneous Rocks
Igneous rocks form when molten material cools and hardens. They may form either below (intrusive) or above (extrusive) the Earth’s surface. They make up most of the rocks observed on Earth. Most igneous rock is buried below the surface and covered with sedimentary rock, so we do not often see just how much igneous rock there is on Earth. In some places, however, large areas of igneous rocks can be seen at Earth’s surface.[219]
A key to understanding the crystalline structure of igneous rocks depends on the rate of cooling. The faster the material cools, the smaller the crystals. If the crystals or the minerals are so small that they can not be identified with the naked eye, we call those aphanitic crystals (think microscopic organisms). If the crystals are large, maybe the size of a pencil eraser, we call those porphyritic crystals.
Classifying Igneous Rocks
Igneous rocks are classified according to how and where they formed and what they are composed of. In other words, igneous rocks are classified based on the following:
➢ whether or not they are plutonic (they cool and crystallize inside the Earth) or volcanic (they cool and crystallize at the surface of the Earth), and
➢ what their mineral composition is; this is described as being felsic, intermediate, or mafic.
Intrusive igneous rocks crystallize deep beneath Earth's surface and if slow cooling occurs, then large crystals can form. Some examples of intrusive igneous rocks are granite, gabbro, diorite, pegmatite, and peridotite. Intrusive igneous rocks are also known as plutonic rocks.
Extrusive igneous rocks erupt onto Earth’s surface and they cool very quickly to form small crystals. In fact, some cool so quickly that they can form into glass. Some examples of extrusive igneous rocks include basalt, andesite, obsidian, pumice, scoria, rhyolite, and tuff. Extrusive igneous rocks are also known as volcanic rocks.
Felsic is a term used to describe igneous rocks that are rich in elements that form quartz and feldspars. An example of a felsic rock is granite. Mafic is a term used to describe igneous rocks that are rich in magnesium and iron. An example of a mafic rock is basalt. Intermediate igneous rocks have compositions between felsic and mafic.
Table 12.2 provides basic information to identify selected igneous rocks.
| Rock Name | Composition | Intrusive or Extrusive | Rock Color | Rock Texture | Other Properties |
|---|---|---|---|---|---|
| Pumice | Felsic | Extrusive | Cream to Gray | Glassy (Frothy) | Will Float in Water |
| Obsidian | Mafic | Extrusive | Dark Brown to Black | Glassy | Known as Nature’s Glass |
| Basalt | Mafic | Extrusive | Gray to Black | Aphanitic | Earth’s Most Abundant Rock |
| Scoria | Mafic | Extrusive | Black to Deep Reds | Aphanitic | Similar to Basalt but Has Bubble-Like Cavities |
| Gabbro | Mafic | Intrusive | Dark Green to Gray | Phaneritic | Most Abundant Rock of the Deep Oceanic Crust |
| Granite | Felsic | Intrusive | Varies | Phaneritic | Oldest and Hardest Rocks Known |
| Rhyolite | Felsic | Extrusive | Pink to Peach | Aphanitic | Silica-rich, Caused by Quick Cooling |
| Diorite | Intermediate | Intrusive | White and Black | Phaneritic | Like a Dalmation, Large Spots of Feldspar and Hornblende Minerals |
| Andesite | Intermediate | Extrusive | Green to Dark Gray | Aphanitic | Usually Found on Earth’s Surface above Subduction Zones |
| Tuff | Intermediate to Felsic | Extrusive | Red to Black | Fragmental | From Large Volcanic Eruptions, the Settling Materials Compact into Cement |
 Check It Out! Igneous Rocks
Check It Out! Igneous Rocks
This lab simplifies igneous rocks, but with some help from National Geographic, a lot more information is available.
Sedimentary Rocks
Transportation is the movement of sediments or dissolved ions from the site of erosion to a site of deposition; this can be by wind, flowing water, glacial ice, or mass movement down a slope. Deposition takes place where the conditions change enough so that sediments being transported can no longer be transported, like when a current slows. Lithification is what happens at depths of hundreds to thousands of meters when those compacted sediments become cemented together to form solid sedimentary rock.[220]
Clastic Sedimentary
A clast is a fragment of rock or mineral, ranging in size from less than a micron (too small to see) to as big as an apartment block. maller clasts tend to be composed of a single mineral crystal, and the larger ones are typically composed of pieces of rock. Most sand-sized clasts are made of quartz because quartz is more resistant to weathering than any other common mineral. Most clasts that are smaller than sand size are made of clay minerals. Most clasts larger than sand size are actual fragments of rock, and commonly these might be fine-grained rock like basalt or andesite, or if they are bigger, coarse-grained rock like granite or gneiss.[221]
- Refer to Table 12.1. What is the hardness of quartz? Do you think its hardness is one reason why quartz is more resistant to weathering than other common minerals? Explain your response in at least one sentence.
Chemical Sedimentary
Whereas clastic sedimentary rocks are dominated by components that have been transported as solid clasts (clay, silt, sand, etc.), chemical sedimentary rocks are dominated by components that have been transported as ions in solution.[222]
The most common chemical sedimentary rock, by far, is limestone. Others include chert, banded iron formations, and a variety of rocks that form when bodies of water evaporate.
Biological processes are important in the formation of some chemical sedimentary rocks, especially limestone and chert. For example, limestone is made up almost entirely of fragments of marine organisms that manufacture calcite for their shells and other hard parts, and most chert includes at least some of the silica tests (shells) of tiny marine organisms such as diatoms.18 When these marine organisms die, their shells sink to the seafloor. After a very long time, these shells are buried, compacted, and cemented into chemical sedimentary rocks.
- Is the sedimentary rock sample shown in Figure 12.9 clastic or chemical. How do you know?

Table 12.3 provides basic information to identify selected sedimentary rocks.
| Rock Name | Clastic or Chemical | Rock Color | Rock Texture |
|---|---|---|---|
| Coquina | Clastic | Tan to Cream | Medium to Coarse |
| Chert | Chemical | Varies | Aphanitic |
| Coal | Chemical | Dark Gray to Black | Banded, but Glassy |
| Shale | Clastic | Blue, Green, Gray | Aphanitic (Mudstone) |
| Conglomerate | Clastic | Varies | Course- Rounded |
| Breccia | Clastic | Varies | Course- Angular |
| Siltstone | Clastic | Gray to Brown | Coarse (Between Shale and Sandstone) |
| Limestone | Clastic | White, Blue to Cream | Coarse (Also Reacts to Acid) |
| Sandstone | Clastic | Varies | Coarse |
| Travertine | Chemical | White to Brown | Crystalline- Result of Mineral Springs |
 Check It Out! Sedimentary Rocks
Check It Out! Sedimentary Rocks
This lab simplifies sedimentary rocks, but with some help from the National Science Foundation, we can observe how clastic sedimentary rocks are formed. Note: Adobe flash player is needed to view this animation.
Metamorphic Rocks
Metamorphism is the change that takes place within a body of rock as a result of being subjected to conditions that are different from those in which it formed. In most cases, but not all, this involves the rock being deeply buried beneath other rocks, where it is subjected to higher temperatures and pressures than those under which it formed. Metamorphism can also take place if cold rock near the surface is intruded by rising magma. Metamorphic rocks typically have different mineral assemblages and different textures from their parent rocks, but they may have the same overall composition.
Foliated
A rock that is foliated means that there is an observable layering or banding. Each layer can be as thin as a sheet of paper or even a meter in width. Imagine such high pressure and temperature that the minerals within a sample are realigned into a banded sequence! Imagine squishing a scoop of ice cream between two cookies.
Non-Foliated
A rock that is non-foliated means that the rock does not have platy or sheet-like structure. These rocks appear as a mass or large uniform piece, such as marble. Imagine such high pressure and temperature that the minerals within a sample can not be aligned or moved. Think of squeezing saltwater taffy. No matter how hard you squish it, the ingredients will never separate, it will just squish!
- Does the metamorphic sample below (Figure 12.10) exhibit foliation or is it non-foliated? How do you know?

Table 12.4 provides basic information to identify selected metamorphic rocks.
| Rock Name | Foliated or Non-Foliated | Rock Color | What Was it Before? |
|---|---|---|---|
| Marble | Non-Foliated | Varies | Limestone |
| Schist | Foliated | Gray but Sparkles | Shale- Mudstone |
| Quartzite | Non-Foliated | White, Pink, to Gray | Sandstone |
| Anthracite | Non-Foliated | Black with Gold Luster | Coal |
| Gneiss | Foliated | Black Stripes with White to Red Filling | Granite |
| Slate | Foliated | Purple to Black | Shale- Mudstone |
| Phyllite | Foliated- Splits Easily into Sheets | Greenish to Black | Shale- Mudstone |
Here is a fun fact: if shale (a sedimentary rock made of compacted clay minerals) undergoes continuous metamorphism, it will transition into four different rocks the longer it undergoes metamorphism. Initially, shale will metamorphose into slate. With increasing heat and pressure, it will then metamorphose into phyllite, then into schist, and lastly into gneiss!
 Check It Out! Metamorphic Rocks
Check It Out! Metamorphic Rocks
This lab simplifies metamorphic rocks, but with some help from the National Science Foundation, we can observe how metamorphic rocks are formed. Note: Adobe flash player is needed to view this animation.
Part D. Rock Identification
How to Use the Basic Rock Identification Tables
The first step is to use the rock dichotomous key shown below to help determine the family of an unknown rock, whether it be igneous, sedimentary, or metamorphic. You will then use the identification tables to determine the name of the specimen (Tables 12.2, 12.3, and 12.4), which are broken into rows and columns. If a specimen has most or all of the properties along a horizontal row, then the name of that sample will be found in the first column on the left.
Rock Dichotomous Key
You can use the following steps to identify a rock sample.
Step 1: Can you see separate mineral crystals in the rock that are randomly intergrown?
➢ If yes, go to step 2.
➢ If no, go to step 7.
Step 2: Is the rock made up of just one kind of mineral?
➢ If yes, go to step 3.
➢ If no, go to step 4.
Step 3: If the rock has crystals of the same mineral, it is probably a metamorphic rock.
Step 4: Are the minerals in a banded or striped pattern?
➢ If yes, go to step 5.
➢ If no, go to step 6.
Step 5. A rock that has a banded pattern is probably a metamorphic rock.
Step 6: A rock with minerals in a mixed pattern is probably an igneous rock.
Step 7: Is the rock full of small air pockets?
➢ If yes, go to step 8.
➢ If no, go to step 9.
Step 8: A rock with small air pockets is probably an igneous rock.
Step 9: Does the rock look like a piece of dark, broken glass?
➢ If yes, go to step 10.
➢ If no, go to step 11.
Step 10: A dark, glassy-looking rock is probably an igneous rock.
Step 11: Is the rock made up of flat plates or sheets?
➢ If yes, go to step 12.
➢ If no, go to step 13.
Step 12: A rock that splits easily into sheets is probably a metamorphic rock.
Step 13: Does the rock have particles in it, like sand, mud, or gravel?
➢ If yes, go to step 14.
➢ If no, go to step 15.
Step 14: A rock that is made of sediment (clay, sand, or pebbles) or fossils is a sedimentary rock.
Step 15: You have a hard rock to identify; try again, and study the rock more carefully before answering each question.
Rock Identification Activity (Hands-On Version)
Using the tools provided, the dichotomous key shown above, and the abbreviated lists of common igneous, sedimentary, and metamorphic rocks (Tables 12.2, 12.3, and 12.4), identify the samples provided by your instructor. Write your answers in question 17 below.
Rock Identification Activity (Digital Version)
Your instructor will indicate which playlist (set 1, 2, or 3) you should use for this activity. Click on the correct QR code or link to access the playlist. Use the dichotomous key shown above and the abbreviated lists of common igneous, sedimentary, and metamorphic rocks (Tables 12.2, 12.3, and 12.4). Write your answers in question 17 below. Note: the videos provided in the playlist do not have audio.
Playlist 1:  Playlist 2:
Playlist 2:  Playlist 3:
Playlist 3: 
Rock Identification Activity: Your Answers
- Identify the rocks.
- Sample __________ (number or letter) is the rock: _______________________
- Sample __________ (number or letter) is the rock: _______________________
- Sample __________ (number or letter) is the rock: _______________________
- Sample __________ (number or letter) is the rock: _______________________
- Sample __________ (number or letter) is the rock: _______________________
- Sample __________ (number or letter) is the rock: _______________________
- Sample __________ (number or letter) is the rock: _______________________
- Sample __________ (number or letter) is the rock: _______________________
- Sample __________ (number or letter) is the rock: _______________________
- Sample __________ (number or letter) is the rock: _______________________
- Sample __________ (number or letter) is the rock: _______________________
- Sample __________ (number or letter) is the rock: _______________________
Part E. Wrap-Up
Consult with your geography lab instructor to find out which of the following wrap-up questions you should complete. Attach additional pages to answer the questions as needed.
- What is the most important idea that you learned in this lab? In two to three sentences, explain the concept and why it is important to know.
- What was the most challenging part of this lab? In two to three sentences, explain why it was challenging. If nothing challenged you in the lab, write about what you think challenged your classmates.
- What is one question that you have about what you learned in this lab? Write your question along with one to two sentences explaining why you think your question is important to ask.
- Review the learning objectives on page 1 of this lab. How would you rate your understanding or ability for each learning objective? Write one sentence that addresses each learning objective.
- Sketch a concept map that includes the key ideas from this lab. Include at least five of the terms shown in bold-faced type.
- Create an advertisement to educate your peers on the important information that you learned in this lab. Include at least three key terms in your advertisement. The advertisement should be about half a page in size (about 4 inches by 6 inches).
- One way to think of physical geography is that it is the study of the relationships among variables that impact the Earth's surface. Select two variables discussed in this lab and describe how they are related.
- How does what you learned in this lab relate to your everyday life? In two to three sentences, explain a concept that you learned in this lab and how it relates to your day-to-day actions.
- How does what you learned in this lab relate to current events?
- Write the title, source, and date of a news item that relates to this lab.
- In two to three sentences, discuss how the news item relates to what you have learned in this lab.
- In one to two sentences, discuss whether or not the news item accurately represents the science that you learned. Tip: consider whether or not the news item includes the complexity of the topic.
- Search O*NET OnLine to find an occupation that is relevant to the topics presented in today's lab. Your lab instructor may provide you with possible keywords to type in the Occupation Quick Search field on the O*NET website.
- What is the name of occupation that you found?
- Write two to three sentences that summarize the most important information that you learned about this occupation.
- What is one question that you would want to ask a person with this occupation?
[205] Text by Steven Earle is licensed by CC BY 4.0
[206] Text by Lumen Learning is licensed under CC BY 4.0
[208] Text by Steven Earle is licensed under CC BY 4.0
[209] Text by Lumen Learning is licensed under CC BY 4.0
[211] Text by Steven Earle is licensed under CC BY 4.0
[213] Text adapted from Wikipedia is licensed under CC BY-SA 3.0
[217] Text by Steven Earle is licensed under CC BY 4.0
[219] Text by Lumen Learning is licensed under CC BY 4.0
[220] Text adapted from Steven Earle is licensed under CC BY 4.0

