5.2: Photosynthesis
- Page ID
- 20860
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Light Energy
The sun emits an enormous amount of electromagnetic radiation (solar energy) that spans a broad swath of the electromagnetic spectrum, the range of all possible radiation frequencies. When solar radiation reaches Earth, a fraction of this energy interacts with and may be transferred to the matter on the planet. This energy transfer results in a wide variety of different phenomena from influencing weather patterns to driving a myriad of biological processes. In Bis2a we are largely concerned with the latter and we discuss some very basic concepts related light and its interaction with biology below.
First, however, we need to review a couple of key properties of light.
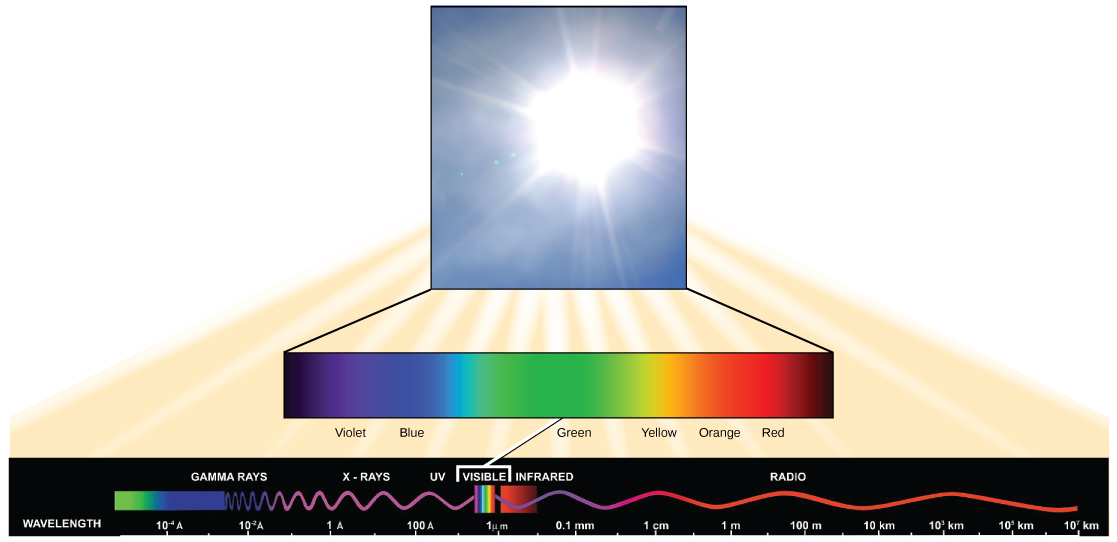
Light has properties of waves. A specific "color" of light has a characteristic wavelength.


Each frequency (or wavelength) of light is associated with a specific energy. The higher the frequency (or shorter the wavelength) the more energy is associated with a specific "color". Wave 2 in the figure above has greater energy than wave 1.
The Light We See
The visible light seen by humans as white light is composed of a rainbow of colors, each with a characteristic wavelength. Certain objects, such as a prism or a drop of water, disperse white light to reveal the colors to the human eye. In the visible spectrum, violet and blue light have shorter (higher energy) wavelengths while the orange and red light have longer (lower energy) wavelengths.

The colors of visible light do not carry the same amount of energy. Violet has the shortest wavelength and therefore carries the most energy, whereas red has the longest wavelength and carries the least amount of energy. (credit: modification of work by NASA)
Absorption by Pigments
The interaction between light and biological systems occurs by several different mechanisms, some of which you may learn about in upper-division courses in cellular physiology or biophysical chemistry. These interactions can initiate a variety of light dependent biological processes that can be grossly grouped into two functional categories: cellular signaling and energy harvesting. Signaling interactions are largely responsible for perceiving changes in the environment (in this case changes in light). An example of a signaling interaction might be the interaction between light and the pigments expressed in an eye. By contrast, light/pigment interactions that are involved in energy harvesting are used for - not surprisingly - capturing the energy in the light and transferring it to the cell to fuel biological processes. In Bis2a we are mostly concerned about this type of interaction of light and biological pigments. Photosynthesis is one example of an energy harvesting interaction.
At the center of the biological interactions with light are groups of molecules we call organic pigments. Whether in the human retina, chloroplast thylakoid, or microbial membrane, organic pigments often have specific ranges of energy or wavelengths that they can absorb. The sensitivity of these molecules for different wavelengths of light is due to their unique chemical makeups and structures. A range of the electromagnetic spectrum is given a couple of special names because of the sensitivity of some key biological pigments: The retinal pigment in our eyes, when coupled with an opsin sensor protein, “sees” (absorbs) light predominantly between the wavelengths between of 700 nm and 400 nm. Because this range defines the physical limits of the electromagnetic spectrum that we can actually see with our eyes, we refer to this wavelength range as the "visible range". However, the sunlight reaching the Earth's surface ranges from the UV into the far infrared. Interestingly, some animals perceive wavelengths that we cannot see. Plants are also very good at perceiving light, and use this information to direct growth (out from under the shade of a taller plant, for example). However, other plant pigments absorb light in order to capture energy. Depending on the plant (or photosynthetic bacterium) the spectrum absorbed by energy-collecting pigments can range from approximately 800 nm (infrared) to approximately 300 nm (the ultraviolet). Green plants carry combinations of pigments that allow them to absorb a wide range of wavelengths, though with varying efficiencies (green plants appear green because they reflect, rather than absorb, most of the photons at a combination of wavelengths that we perceive as green).
Quick question
If a plant were able to absorb 100% of incident photons, what color would it be? What if a variant of the same species of plant lacked a pigment required to absorb red light?
Three Key Types of Pigments We Discuss in Bis2a
Chlorophylls
Chlorophylls (including bacteriochlorophylls) are part of a large family of pigment molecules. There are five major chlorophyll pigments named: a, b, c, d, and f. Chlorophyll a is related to a class of more ancient molecules found in bacteria called bacteriochlorophylls. Chlorophylls are structurally characterized by ring-like porphyrin group that coordinates a metal ion. This ring structure is chemically related to the structure of heme compounds that also coordinate a metal and are involved in oxygen binding and/or transport in many organisms. Different chlorophylls are distinguished from one another by different "decorations"/chemical groups on the porphyrin ring.
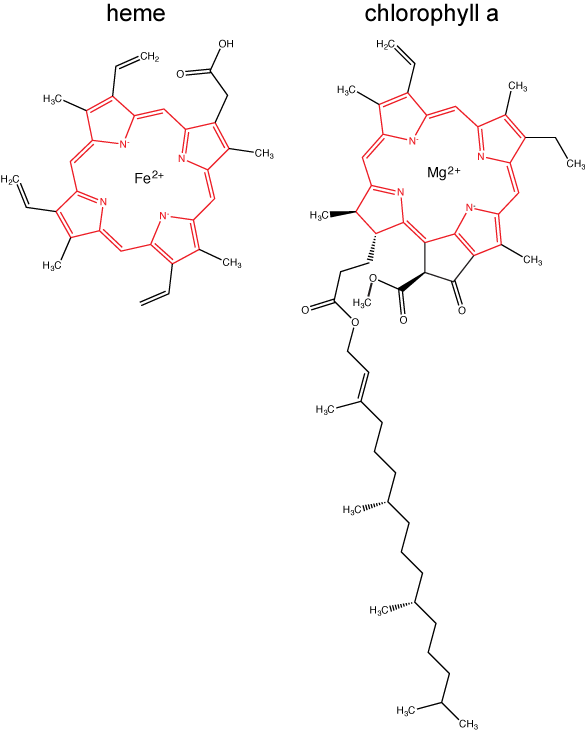
The structure of heme and chlorophyll a molecules. The common porphyrin ring is colored in red. Attribution: Marc T. Facciotti (original work)
Carotenoids
Carotenoids are the red/orange/yellow pigments found in nature. They are found in fruit — such as the red of tomato (lycopene), the yellow of corn seeds (zeaxanthin), or the orange of an orange peel (β-carotene, below) — and can be used as biological "advertisements" to attract seed dispersers (animals or insects that may carry seeds elsewhere). In photosynthesis, various carotenoids can function as either light-harvesting or protective (energy-dispersing) pigments in photosynthetic reaction centers.
Each type of pigment can be identified by the specific pattern of wavelengths it absorbs from visible light. This characteristic is known as the pigment's absorption spectrum. The graph in the figure below shows the absorption spectra for chlorophyll a, chlorophyll b, and a carotenoid pigment, called β-carotene. Notice how each pigment has a distinct set of peaks and troughs, revealing a highly specific pattern of absorption. These differences in absorbance are due to differences in chemical structure (some are highlighted in the figure). Chlorophyll a absorbs wavelengths from either end of the visible spectrum (blue and red), but not green. Because green is reflected or transmitted, chlorophyll appears green. Carotenoids absorb in the short-wavelength blue region, and reflect the longer yellow, red, and orange wavelengths.
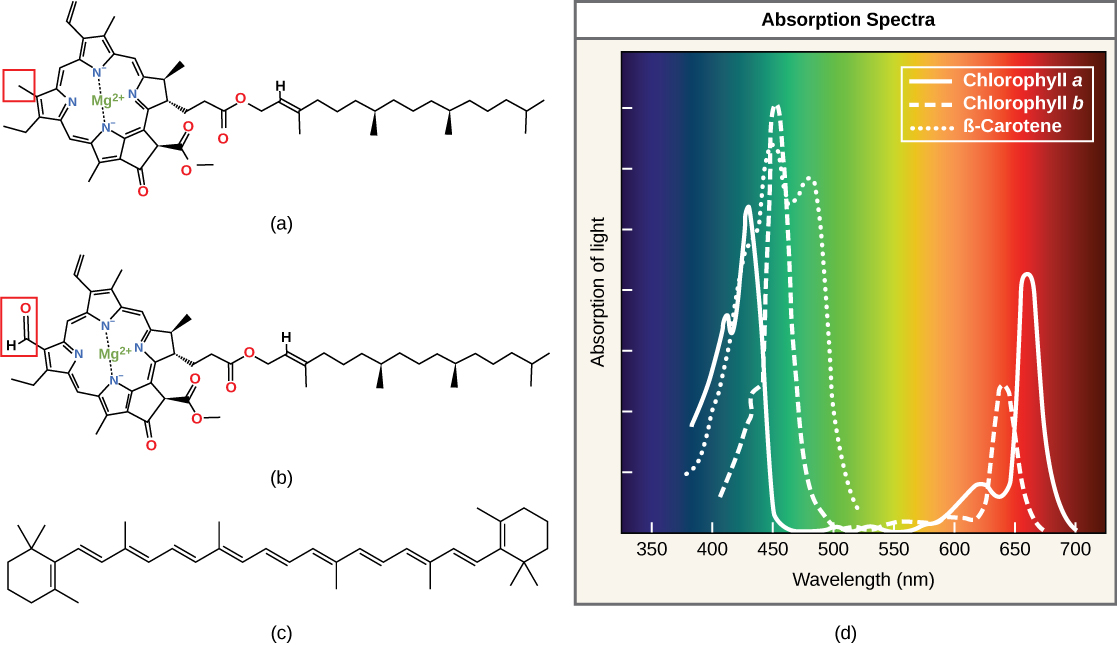
(a) Chlorophyll a, (b) chlorophyll b, and (c) β-carotene are hydrophobic organic pigments found in the thylakoid membrane. Chlorophyll a and b, which are identical except for the part indicated in the red box, are responsible for the green color of leaves. Note how the small amount of difference in chemical composition between different chlorophylls leads to different absorption spectra. β-carotene is responsible for the orange color in carrots. Each pigment has (d) a unique absorbance spectrum.
Importance of having multiple different pigments
Not all photosynthetic organisms have full access to sunlight. Some organisms grow underwater where light intensity and available wavelengths decrease and change, respectively, with depth. Other organisms grow in competition for light. Plants on the rainforest floor for instance must be able to absorb any bit of light that comes through, because the taller trees absorb most of the sunlight and scatter the remaining solar radiation. To account for these variable light conditions, many photosynthetic organisms have a mixture of pigments whose expression can be tuned to improve the organism's ability to absorb energy from a wider range of wavelengths than would be possible with one pigment alone.
What happens when an atom absorbs a photon?
When an atom absorbs a photon of light, an electron acquires that energy, leaving its ground (lowest potential energy) orbital and moving up to a higher energy orbital. This is an unstable situation.

What are the fates of the "excited" electron? There are a few possible outcomes, which are schematically diagrammed in the figure below. These options are:
- (I and II below) The electron can relax to a lower quantum state (back to the ground state), transferring energy as heat or light (a photon of less energy than the incident photon; the wavelength of this "fluoresced" photon is determined by the specific difference in energy between the excited state orbital and the ground state orbital).
- (III below) The energy can be transferred by resonance to a neighboring molecule as the e- returns to a lower quantum state. There is virtually no loss of energy in this transfer.
- (IV below) Because the energy changed the reduction potential such that the molecule is now a stronger e- donor, this high-energy e- can be transferred exergonically to an appropriate e- acceptor. In other words, the excited state can be involved in a redox reactions. This is a photochemical reaction.

Photophosphorylation: an Overview
Photophosphorylation is the process of transferring the energy from light into ATP. The evolutionary roots of photophosphorylation are likely in the anaerobic world, between 3 billion and 1.5 billion years ago, when life was abundant in spite of the absence of molecular oxygen. Photophosphorylation probably evolved relatively shortly after electron transport chains and anaerobic respiration. The first step of the process involves the absorption of a photon by a pigment molecule. Light energy is transferred to the pigment and promotes electrons into an excited state. (Note the use of anthropomorphism here, the electrons are not suddenly hopping all over or celebrating their promotion. They are simply in a higher energy orbital. This is in contrast to the pigment in its "unexcited", or "ground state".) While in the excited state, the pigment has a much lower reduction potential (E˚', it moves upward on our electron tower, it becomes a stronger reducing agent) and can donate these unstable, high potential energy electrons to carriers with greater E˚' - carriers that the ground state pigment would not be capable of reducing. We have now gotten the ball rolling- we have a highly reducing electron that we can pass from carrier to carrier (as in respiration's electron transport chain).
A quick example: For example, as you can see in the Table below, the ground state pigment at the reaction center of PSII (the "chlorophyll a" in P680) cannot reduce anything listed in the table- it is the weakest reducing agent described there (even weaker than H2O!). However, once it is has absorbed a photon of sufficient energy to boost P680 into its excited (*) state, it becomes a strong reducing agent, capable of reducing Pheophytin.
As electrons pass from one electron carrier to another via redox reactions, these exergonic transfers could be coupled to the endergonic transport (or "pumping") of protons across a membrane to create an electrochemical gradient (as we saw in respiration). This electrochemical gradient generates a proton motive force whose concentration gradient can then be coupled to the endergonic production of ATP, via ATP synthase (again, just as in respiration). Electrons that have been used to generate the proton gradient are returned to an oxidized chlorophyll reaction center, restoring its missing e-.
However, the electron energized by light might have an alternative fate: it might descend through a different series of carriers (without pumping protons) and instead be deposited onto a close relative of NAD+ called NADP. Addition of 2 e- generates NADPH, which is going to be used to build sugars from CO2. Since the e- is not recycled to a pigment, the original oxidized pigment must regain an electron from somewhere else. This electron must come from an external source with a lower reduction potential than the (ground state) pigment and depending on the reduction potential of that pigment there are different possible sources that might be employed, including H2O, reduced sulfur compounds such as SH2, and even elemental S0.
This is all very complicated, and was introduced rather quickly, so this provides you with an ideal opportunity to attempt to sketch this process, just to see what you do and don't know. Write down your "issues". This may be very challenging. While you read the text below, you can see how good your guess was. It's always easier to learn when you know what exactly you don't know!
If your drawing is done, let's go over this more specifically and carefully.

Cyclic Photophosphorylation (an example)
Although Green sulfur bacteria live in the absence of visible light, they can perform photosynthesis using remarkably low-energy infrared photons. In the cyclic photophosphorylation depicted below, bacteriochlorophyllred at the reaction center (called P840, because it has an absorption maximum at a wavelength of 840 nm) absorbs a photon, energizing an electron to the extent that it can now be transferred to the next electron carrier in an electron transport chain (here an iron-sulfur protein). In an electron transport chain analogous to that of respiration, the electron is passed exergonically from carrier to carrier. The redox reaction at one of the carriers powers a proton pump, pushing protons into a higher concentration compartment. Eventually the electron is used to reduce bacteriochlorophyllox (making a complete loop) and the whole process can start again. This flow of electrons is cyclic and is therefore said to drive "cyclic photophosphorylation". The "phosphorylation" occurs when ATP is produced from ADP when the highly concentrated protons are allowed to reenter the cytosol via ATP synthase (as in respiration).

Cyclic electron flow. The reaction center P840 absorbs light energy and becomes excited, (the excited form is not illustrated here- where would you place it on this axis?). The excited electron is ejected and used to reduce an FeS protein leaving an oxidized reaction center. The electron is transferred to a quinone, then to a series of cytochromes which in turn reduce the P840 reaction center. The process is cyclical. Note the gray arrow coming from the FeS protein going to a ferridoxin (Fd), also in gray. This represents an alternative pathway the electron can take and will be discussed below in non-cyclic photophosphorylation.
Suggested discussion
The figure of cyclic photophosphorylation above depicts the flow of electrons in a respiratory chain. How does this process help generate ATP? The schematic in the figure above demonstrates how cyclic electron flow works, but does not include P840* (excited state P840). Nor does it illustrate proton pumping (by the cytochrome bc1 complex) or the synthesis of ATP. Try drawing a schematic that includes these processes also. You might want to draw two figures- one with an E˚' axis (as drawn above), and another that shows the locations of these activities in the bacterial inner membrane. As always, try doing this without looking to other resources first. This will help you pinpoint any knowledge "potholes"- make a list of those, and then fix them.
Non-cyclic photophosphorylation (another example from Green sulfur bacteria)
In cyclic photophosphorylation electrons cycle from reduced bacteriochlorophyll through a series of electron carriers and eventually back to oxidized bacteriochlorophyll. There is, theoretically, no net loss of electrons; they stay in the system. In non-cyclic photophosphorylation electrons are removed from the photosystem, as they end up in NADPH after moving through an electron transport chain (which does not pump protons- there's only so much you can ask from the energy of a single photon). This NADPH will be used for carbon fixation. Thus in order to keep generating NADPH there's needs to be a source of electrons to refill the "electron hole" in bacteriochlorophyll. This source must a lower (= higher up on our chart) reduction potential than bacteriochlorophyll itself. An electron tower is provided above so you can see what compounds might potentially be used to reduce the oxidized form of bacteriochlorophyll. You can see that water is not an option as a source of electrons for P840ox, as water is too weak a reducing agent to donate an electron to that molecule. Whether an organism can actually employ a particular reducing agent will depend on what catalysts (enzymes) it can produce, as well as the energetics of the metabolites. Green sulfur bacteria have evolved to live in H2S rich environments, and can utilize H2S as an electron donor. Green plants, in contrast, can't do this. Thus for photosynthesis to occur, green sulfur bacteria must find this metabolizable source of electrons.
Non-cyclic Electron Flow

Non-cyclic electron flow. In this example, the P840 reaction center absorbs light energy and becomes energized, the emitted electron reduces an FeS protein and in turn reduces ferredoxin. Reduced ferredoxin (Fdred) can now reduce NADP to form NADPH. The electrons are now removed from the system as NADPH, finding their way to carbon fixation or other anabolic reactions. The electrons need to be replaced on P840, which requires an external electron donor. In this case, H2S can serve as the external electron donor.
It should be noted that for these bacterial photophosphorylation pathways, for each electron donated from a reaction center the resulting output from that electron transport chain is either the formation of NADPH (which requires 2 electrons) or ATP, not both. In other words, there are two possible paths that the electrons could take "downstream" of P840*. The cell will choose to run one or the other pathway depending on its immediate needs.
Oxygenic Photophosphorylation
Generation of NADPH and ATP
The overall function of "light-dependent" reactions of photosynthesis is to transform solar energy into chemical compounds, in the form of NADPH and ATP. This energy supports the "light-independent" reactions and fuels the assembly of sugar molecules. As illustrated above for green sulfur bacteria, protein complexes and pigment molecules will work together to produce NADPH and ATP. To quickly review what we discovered above, the absorbance of a photon makes all the difference. Normally, a good oxidizing agent (P840ox, capable of oxidizing H2S) would, in its reduced form, be a relatively poor reducing agent (incapable of reducing NADP+). The energy added by absorbance of a photon- even, for green sulfur bacteria, a relatively weak infrared photon- turns this weak reducing agent into a strong one, at least until that energy is lost. The temporary addition of energy to P840red makes it a good electron donor, loss of that energy (with the electron) allows it to be a good oxidizing agent, and the net result is that electrons are transferred from H2S to NADP+, provided light is available.
As you will see, oxygenic photosynthesis is more complex than the "sulfur-genic" photosynthesis described above, requiring two different reaction centers, with different reduction potentials. While this complexity is inconvenient for students (and instructors) it is extremely convenient for the bacteria and plants that employ it as they can derive electrons from an unlimited supply: water. The complexity of oxygenic photosynthesis was the only evolutionary solution available to the Grand Design Challenge: "What biological pigment can both oxidize water and, when activated by light, reduce NADP+?" As you'll see, Nature's answer was "no can do- why not use two?".
Parts of the discussion below should sound extremely familiar if you have followed the discussion above. Try to see of you can determine where there are differences between photosynthesis as performed by green sulfur bacteria and as performed by cyanobacteria and green plants.
Suggested discussion
Step back a little. Why is it a reasonable goal to want to make NADPH and ATP? In the discussion of glycolysis and the TCA cycle the goal was also to make ATP and NADH. What is the key difference between these two processes (no, it's not the P).
Why is it a convenient to be able pull electrons from water to make NADPH?

As in the green sulfur bacteria example above, the step that transfers light energy into the biomolecule takes place in a multiprotein, multipigment complex called a photosystem. In the green sulfur bacterium, an electron from a single type of photosystem, carrying the pigment P840, could be used to power either the formation of NADPH or the formation of ATP, depending on ther outhe of the electron transport chain, which is regulated by the needs of the bacterium.
In oxygenic photosynthesis, two types of pigment are found embedded in the thylakoid membrane (in plants) or the bacterial inner membrane (in cyanobacteria). These two types are simply called photosystem II (PSII, carrying P680) and photosystem I (PSI, carrying P700), and were named (confusingly) in the order of their discovery. The two complexes differ on the basis of what they oxidize (that is, their source of electrons) and what they reduce (the place to which they deliver their energized electrons). Working in tandem, these two photosystems can power the production of both NADPH and ATP.
Both photosystems have the same basic structure; a number of antenna proteins to which chlorophyll molecules are bound surround the reaction center where the photochemistry takes place. Each photosystem is serviced by this light-harvesting complex, which passes energy from sunlight to the reaction center; it consists of multiple antenna proteins that contain a mixture of 300–400 chlorophyll a and b molecules as well as other pigments like carotenoids. The absorption of a single photon by any of the chlorophylls pushes that molecule into an excited state. In short, the light energy has now been captured by biological molecules but is extremely unstable and not yet stored in any useful form. The captured energy is transferred from chlorophyll to chlorophyll until ...eventually... (after about a millionth of a second), it is delivered to the reaction center. Up to this point, only energy has been transferred between molecules, not electrons. In other words, no new bonds have formed.
Recall/consider: Both the reaction center and the antennae pigments contain chlorophyll a. What is the essential difference between and antennae chlorophyll a and reaction center chlorophyll a?

The reaction center contains a pair of chlorophyll a molecules with a special property. Those two chlorophylls are located close to an oxidizing agent and so can undergo oxidation (upon excitation). It is at this step that light energy is transformed into the more stable form of chemical energy. All of the subsequent redox reactions are involved in pumping protons or in delivering that e- to NADP. Once reduced, this "reducing power" carrier, as well that the newly constructed ATP, will participate in the Calvin cycle where the electron can be deposited onto CO2 for long-term storage in the form of a carbohydrate. The Calvin cycle takes place in the stroma, conveniently located vis a vis the ATP- and NADPH-producing thylakoids

The chloroplast (image by Kelvinsong) looks like a bacterium but is actually an intracellular organelle descended from cyanobacteria. It has an outer membrane and and inner membrane, which enclose the smaller stacks of membrane-bound thylakoids. The photosynthetic pigments and ATP synthetic apparatus are embedded in the thylakoid membranes; carbon fixation (the Calvin Cycle) occurs in the stroma: the aqueous compartment that surrounds the thylakoids.
The electron transport chain
Refer to the diagram below of the "Z scheme" (you will have to turn it sideways to see the "Z"). If you have already drawn your own diagram, fantastic- you can check it against this one.
The reaction center of PSII (called P680) delivers its high-energy electrons, one at a time, to a primary electron acceptor, and these pass through the electron transport chain (Plastoquinones to cytochrome complex to plastocyanin) to the oxidized chlorophyll in P700ox. Along the way, the cytochrome complex pumps protons across the thylakoid membrane (into the lumen) to generate a proton gradient, the energy of which will eventually be harvested for ATP synthesis.
P680ox’s missing electron is replaced by extracting an electron from water. Splitting one H2O molecule releases two electrons, two hydrogen atoms, and one atom of oxygen. Splitting two molecules of water is required to form one molecule of diatomic O2 gas. For this reason, the water-splitting apparatus of PSII carries a manganese core that gradually releases electrons, one by one, to refill the electron "hole" in P680. PSII becomes repeatedly oxidized, the Mn atoms continuously "reload" the pigment. When these metal ions are depleted of a total of 4 electrons, they become oxidizing enough to actually rip electrons from 2 molecules of water, releasing 4 protons and an O2. In plants, about 10 percent of that O2 is used by mitochondria in the leaf to support oxidative phosphorylation (respiration). The remainder escapes to the atmosphere as waste, where it is picked up by aerobic organisms to support their own aerobic respiration.
As electrons move through the proteins that reside between PSII and PSI, they take part in exergonic redox transfers. The free energy associated with the exergonic redox reaction is coupled to the endergonic transport of protons from the stromal side of the membrane to the thylakoid lumen. Those hydrogen ions, plus the ones produced by splitting water, accumulate in the thylakoid lumen and create a proton motive force that will be used to drive the synthesis of ATP in a later step.
The electrons, reaching the end of that electron transport chain are then deposited on PSI(P700)ox, in their ground state. But there's no rest for the weary- they are then re-energized by light, reloaded onto another ETC, and are sufficiently energetic to be donated to NADP+ at the terminus of that chain. As described above for green sulfur bacteria, the NAPDH is employed for other reductive reactions. Therefore, to complete this process another electron is delivered to PSI via the ETC that originates with PSII. That energy is transferred to the PSI reaction center (called P700). P700 is oxidized and sends an electron through several intermediate redox steps to NADP+ to form NADPH. Thus, PSII captures the energy in light and couples its transfer via redox reactions to the creation of a proton gradient. As already noted, the exergonic and controlled relaxation of this gradient can be coupled to the synthesis of ATP. PSI captures energy in light and couples that, through a series of redox reactions, to reduce NADP+ into NADPH. The two photosystems work in concert to simultaneously produce ATP and NADPH. However, if the cell needs more ATP vs. NADPH, photophosphorylation can be decoupled from NADPH production by routing PSI's electron through the proton-pumping cyt bf complex, rather than directing it to NADP.
What if: the electron from the PSII to PSI ETC arrived at PSI and found that the molecule was already reduced?
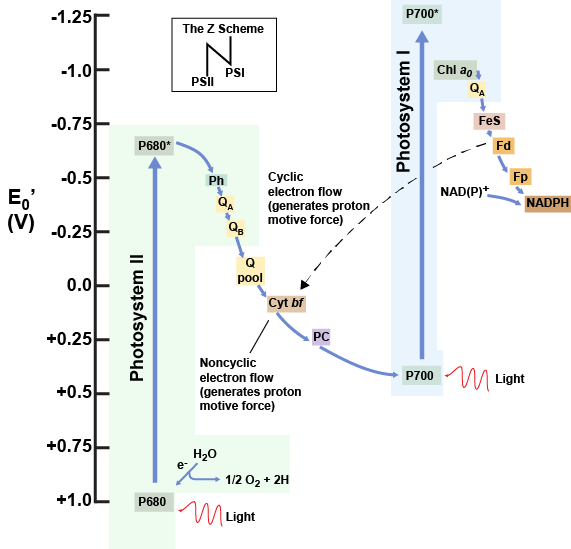
A diagram depicting the flow of electrons and the reduction potentials of their carriers in oxygenic photosynthetic systems expressing both photosystem I (contains P700) and photosystem II (contains P680). The dotted line indicates a cyclic alternative route, which does not produce NADPH, but does pump protons.
Attribution: Marc T. Facciotti (own work)
Suggested discussion
A major difference between the green sulfur bacterium light-dependent reactions and the green plant's "Z scheme" light-dependent reactions is that the noncyclic green sulfur bacterium's system only makes NADPH, while the Z scheme's noncyclic light reactions make both NADP and ATP. Discuss what the differences there might be between the two systems, that allow the green plant system to make both types of energy-rich molecules?
Light Independent Reactions and Carbon Fixation
A short introduction
The general principle of carbon fixation is that some cells under certain conditions can take CO2 and reduce it to a usable cellular form. Most of us are aware that green plants can take up CO2 and produce O2 in during photosynthesis. We have just discussed the ability of a cell to transfer light energy onto chemicals and ultimately to produce the energy carriers ATP and NADPH in a process known as the light reactions. In photosynthesis, the plant cells use the ATP and NADPH formed during photophosphorylation to reduce CO2 to sugar, (as we will see, specifically G3P, an intermediate in glycolysis also) in what are called the dark reactions. While we appreciate that this process happens in green plants, photosynthesis had its evolutionary origins in the bacterial world. In this module we will go over the general reactions of the Calvin Cycle, a reductive pathway that incorporates CO2 into cellular material.
In photosynthetic bacteria such as Cyanobacteria and purple non-sulfur bacteria, as well as in plants, the energy (ATP) and reducing power (NADPH) obtained from the light reactions is coupled to "Carbon Fixation", the incorporation of inorganic carbon (CO2) into organic molecules; initially as glyceraldehyde-3-phosphate (G3P) and eventually into glucose. Organisms that can obtain all of their required carbon from an inorganic source (CO2) are referred to as autotrophs, while those organisms that require organic sources of carbon, such as glucose or amino acids, are referred to as heterotrophs. The biological pathway that leads to carbon fixation in green plants and cyanobacteria is called the Calvin Cycle and is a reductive pathway (consumes energy and electrons) which leads to the reduction of CO2 to G3P. There are at least five other pathways for carbon fixation, used by other autotrophic prokaryotes.
The Calvin Cycle: the reduction of CO2 to Glyceraldehyde 3-Phosphate

Light reactions harness energy from the sun to produce chemical bonds, ATP, and NADPH. A stylized chloroplast is shown above. These energy-carrying molecules are made in the stroma where carbon fixation (here the Calvin Cycle, drawn in an extremely simplified form on the right) takes place.
In plant cells, the Calvin cycle takes place in the stroma (outside of the thylakoids) of the chloroplasts. While the process is similar in cyanobacteria, there are no specific organelles that house the Calvin Cycle and the reactions occur in the cytoplasm around a complex intracellular membrane system derived from the plasma membrane. There is strong evidence that supports the hypothesis that the origin (actually, several independent origins) of chloroplasts was from a symbiosis between cyanobacteria and nonphotosynthetic cells.
Note that in Dr. Britt's class you will not have to memorize the Calvin cycle; you only need to know what goes in, the first step, and what comes out. Dr. Britt suggests this video, which may lack style, but it's clear on the basics.
Stage 1: Carbon Fixation
In the stroma of plant chloroplasts, in addition to CO2,two other components are present to initiate the light-independent reactions: an enzyme called ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), and ribulose bisphosphate (RuBP), as shown in the figure below. Ribulose-1,5-bisphosphate (RuBP) is composed of five carbon atoms and includes two phosphates.

RuBisCO (ribulose bis phosphate carboxylase) catalyzes a reaction between CO2 and RuBP. For each CO2 molecule that reacts with one RuBP, a 6-Carbon molecules is formed. The resulting 6 C molecule will immediately split to form two molecules of another 3-carbon compound (3-PGA). Note that we have added only one C (Carbon) to an existing 5 C chain to make these 2 3-C molecules! Thus we will have to run this initial phase three times to "bleed off" a 3-C and regenerate the 3 RuBP's that were employed to capture each CO2.
Stage 2: Reduction
ATP and NADPH are used to convert the six molecules of 3-PGA into six molecules of a 3C molecule: glyceraldehyde 3-phosphate (G3P) - a carbon compound that you might or might not remember from glycolysis. Six molecules of both ATP and NADPH are used up in the process, helping to drive the reactions and produce the electrons required to reduce the incoming CO2. The "spent" molecules (ADP and NADP+) return to the nearby thylakoids to be recycled back into ATP and NADPH.
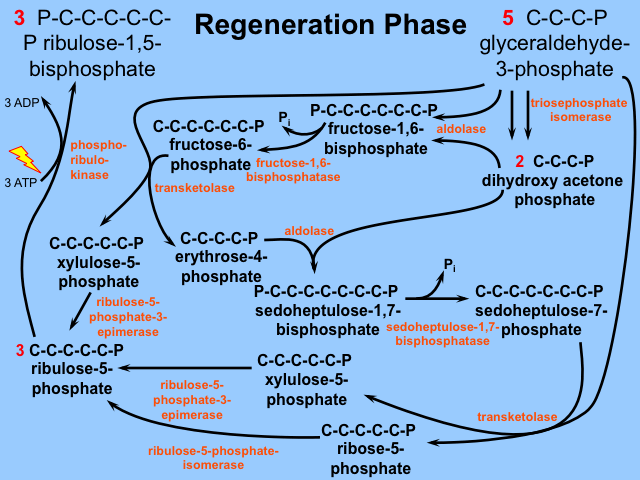
Stage 3: Regeneration
Remember that we have consumed 3 molecules of RuBP to bleed off one G3P. Thus we need to somehow regenerate these 3 RuBP from the remaining 5 G3Ps. This regeneration phase might be politely described as "interesting", or more colloquially as "A Frigging Nightmare". Three more molecules of ATP are used in these regeneration reactions. It is included for your enjoyment below. Again, Dr. Britt will only quiz you on what goes into the Calvin cycle, its first step, and what comes out.