5.3: Respiration
- Page ID
- 20862
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Respiration Overview
In the next few lectures we start to learn about the process of respiration and the roles that electron transport chains play in this process. A definition of the word "respiration" that most people are familiar with is "the act of breathing". When we breath, air-including molecular oxygen- is brought into our lungs from outside of the body, the oxygen then becomes reduced, and waste products, including the reduced oxygen in the form of water, are exhaled. Simply put, some reactant comes into the organism and then gets reduced and leaves the body as a waste product. This generic idea can be generally applied across biology and oxygen need not always be the compound that brought in, reduced, and dumped as waste. These compounds where the electron is finally "dumped" are more generally known as "terminal electron acceptors". The molecules from which the electrons originate can vary greatly across biology (we have looked at one common source - the reduced carbon-based molecule glucose). We will often refer to these molecules as "fuel", and the analogy with the burning of gasoline, propane, or coal is quite apt; these are all simply sources of electrons, and the waste product is CO2 and water. But once again, keep in mind that many organisms can use non-carbon based sources of electrons, and transfer the electrons to other kinds of oxidizing agents.
In between the original electron source and the terminal electron acceptor are a series of biochemical reactions involving at least one redox reaction (during the course of glycolysis, pyruvate oxidation, and the TCA cycle we saw many!). These redox reactions harvest energy for the cell by coupling exergonic redox reactions to an energy-requiring reactions in the cell. In respiration, a special set of enzymes carry out a linked series of redox reactions that ultimately transfer electrons to the terminal electron acceptor. These "chains" of redox enzymes and electron carriers are called electron transport chains (ETC). ETCs are therefore the portion of respiration that use an electron acceptor (brought in from outside of the cell) as the final/terminal acceptor for the electrons that were removed from the intermediate compounds in catabolism. In aerobically respiring eukaryotic cells the ETC is composed of four large, multiprotein complexes embedded in the inner mitochondrial membrane and two small diffusible electron carriers shuttling electrons between them. The electrons are passed from enzyme to enzyme through a series of redox reactions. These reactions couple the exergonic redox transfers to the endergonic transport of hydrogen ions across the membrane. This process contributes to the creation of a transmembrane electrochemical gradient. The electrons passing through the ETC gradually lose potential energy, and are finally deposited on the terminal electron acceptor, which is removed as waste from the cell. When oxygen is the final electron acceptor, the free energy difference of this multistep redox process- from the 1st step in the ETC to the last- is ~ -60 kcal/mol when NADH donates electrons or 45 kcal/mol when FADH2 donates electrons.
Introduction to redox, oxidative phosphorylation and Electron Transport Chains
In a prior module (Cashing in on Redox) we discussed the general concept of redox reactions in biology and introduced the Electron Tower, a tool to help you understand redox chemistry and to estimate the direction and magnitude of potential energy differences for various redox couples. In earlier modules, substrate level phosphorylation and fermentation were discussed and we saw how exergonic redox reactions could be directly coupled by enzymes to the endergonic synthesis of ATP. These processes are hypothesized to be one of the oldest forms of energy production used by cells. In this section we discuss the next evolutionary advancement in cellular energy metabolism, oxidative phosphorylation of ADP to form ATP. First and foremost, oxidative phosphorylation does not necessarily imply the use of oxygen as the terminal electron acceptor. Oxidative phosphorylation evolved before O2 was available, and has continued to evolve since then. As "obligate aerobes" (organisms that can't live without O2) we ourselves can only use O2 as the terminal acceptor. Some organisms are far more flexible, metabolically, and others (obligate anaerobes) can't use O2 at all. The process of generating ATP from ADP is called oxidative phosphorylation (vs. the substrate level phosphorylation seen earlier, in glycolysis and the citric acid cycle) because it relies on redox reactions to generate a electrochemical transmembrane potential that can then be used by the cell to add a phosphate group to ADP (or to do other kinds of work). If you think about it, these are not the most appropriate names, as redox reactions occur in glycolysis and the citric acid cycle too.
A quick summary of Electron Transport Chains
The ETC begins with the donation of electrons from NADH, FADH2 or other reduced compounds to a first acceptor in the chain. The electron moves through a series of electron transporters, carriers (mostly proteins) that undergo a series of redox reactions. The free energy transferred from these exergonic redox reactions is, in some steps, coupled to the endergonic movement of protons across a membrane. Since the membrane is an effective barrier to ions, this pumping results in an unequal accumulation of protons across the membrane. This in turn "polarizes" or "charges" the membrane, with a net positive (protons) on one side of the membrane and a negative charge on the other side of the membrane. The separation of charge creates an electrical potential. In addition, the accumulation of protons also causes a pH gradient known as a chemical potential across the membrane (the protons are being pumped from a low concentration to a higher [H+] environment). Together these two gradients (electrical and chemical) are called an electro-chemical gradient. The -∆G of the electrons moving down the ETC is harvested in the form of this gradient.
Review: The Electron Tower
Since redox chemistry is so central to the topic we begin with a quick review of the table of reduction potentials - sometimes called the "redox tower" or "electron tower". You may hear your instructors use these terms interchangeably. As we discussed in previous modules, all kinds of compounds can participate in biological redox reactions. Making sense of all of this information and ranking potential redox pairs can be confusing. A tool has been developed to rate redox half reactions based on their reduction potentials or E0' values. Whether a particular compound can act as an electron donor (reductant) or electron acceptor (oxidant) depends on what other compound it is interacting with. The redox tower ranks a variety of common compounds (their half reactions) from most negative E0', compounds that readily get rid of electrons, to the most positive E0', compounds most likely to accept electrons. The tower organizes these half reactions based on the ability of comopunds to accept electrons. In many redox towers each half reaction is written by convention with the oxidized form on the left followed by the reduced form to its right. The two forms may be either separated by a slash, for example the half reaction for the reduction of NAD+ to NADH is written: NAD+/NADH (2e-), or by separate columns. An electron tower is shown below.

Note
Use the redox tower above as a reference guide to orient you as to the reduction potential of the various compounds. Redox reactions may be either exergonic or endergonic depending on the relative redox potentials of the donor and acceptor (represented by two different half reactions on the tower). Also remember there are many different ways of looking at this conceptually; this type of redox tower is just one way.
Note: Language shortcuts reappear
In the redox table above some entries seem to be written in unconventional ways. For instance Cytochrome cox/red. There only appears to be one form listed. Why? This is another example of a language shortcut (probably employed so it fits in the column) that can be confusing. The notation above could be rewritten as Cytochrome cox/Cytochrome cred to indicate that the cytochrome c protein can exist in either an oxidized state (Cytochrome cox ) or reduced state (Cytochrome cred).
Review Redox Tower Video
For a short video on how to use the redox tower in redox problems click here. This video was made by Dr. Easlon for Bis2A students.
Using the redox tower: A tool to help understand electron transport chains
By convention the tower half reactions are written with the oxidized form of the compound on the left and the reduced form on the right. Notice that compounds such as glucose and hydrogen gas are excellent electron donors and have very low reduction potentials E0'. Compounds, such as oxygen and nitrite, whose half reactions have relatively high positive reduction potentials (E0') generally make good electron acceptors and are found at the opposite end of the table.
Example: Menaquinone
Let's look at menaquinoneox/red. This compound sits in the middle of the redox tower with an half-reaction E0' value of -0.074 eV. Menaquinoneox can spontaneously (ΔG<0) accept electrons from reduced forms of compounds with lower half-reaction E0'. Such transfers form menaquinonered and the oxidized form of the original electron donor. In the table above, examples of compounds that could act as electron donors to menaquinone include FADH2, an E0' value of -0.22, or NADH, with an E0' value of -0.32 eV. Remember the reduced forms are on the right hand side of the red/ox pair.
Once menaquinone has been reduced to menaquinone(red), it can now spontaneously (ΔG<0) donate electrons to any compound with a higher half-reaction E0' value. Possible electron acceptors include cytochrome box with an E0' value of 0.035 eV; or ubiquinoneox with an E0' of 0.11 eV. Remember that the oxidized forms lie on the left side of the half reaction.
Electron Transport Chains
An electron transport chain, or ETC, is composed of a group of protein complexes in and around a membrane that help energetically couple a series of exergonic/spontaneous redox reactions to the endergonic pumping of protons across the membrane to generate a an electrochemical gradient. This electrochemical gradient creates a free energy potential that is termed a proton motive force whose energetically "downhill" exergonic flow can later be coupled to a variety of cellular processes.
ETC Overview
Step 1. Electrons enter the ETC from an electron donor, such as NADH or FADH2 which are generated during a variety of catabolic reactions, including those associated with glucose oxidation. Depending on the complexity (number and types of electron carriers) of the ETC being used by an organism, electrons can enter at a variety of places in the electron transport chain - this depends upon the respective reduction potentials of the proposed electron donors and acceptors. In some metabolically flexible organisms, the chain could also terminate at a variety of external electron acceptors.
Step 2. After the first redox reaction, the initial electron donor will become oxidized and the electron acceptor will become reduced. The difference in redox potential between the electron acceptor an donor is related to ΔG by the relationship ΔG = -nFΔE, where n = the number of electrons transferred and F = Faraday's constant. The larger a positive ΔE the more exergonic a reaction.
Step 3. If sufficient energy transferred during an exergonic redox step the electron carrier may couple this negative change in free energy to the endergonic process of transporting a proton from one side of the membrane to the other.
Step 4. After multiple redox transfers, the electron is delivered to a molecule known as the terminal electron acceptor. In the case of humans, the terminal electron acceptor is oxygen. However, there are many, many, many, other possible electron acceptors, see below.
Note
Electrons entering the ETC do not have to come from NADH or FADH2. Many other compounds can serve as electron donors, the only requirements are that there exists an enzyme that can oxidize the electron donor and then reduce another compound and that the E0' be positive (e.g. ΔG<0). Even a small amounts of free energy transfers can add up. For example there are bacteria that use H2 as an electron donor. This is not too difficult to believe because the half reaction 2H+ + 2 e-/H2 has a reduction potential (E0') of -0.42 V. If these electrons are eventually delivered to oxygen then the ΔE0' of the reaction is 1.24 V which corresponds to a large negative ΔG (-ΔG). Alternatively, there are some bacteria that can oxidize iron, Fe2+ at pH 7 to Fe3+ with a reduction potential (E0') of + 0.2 V. These bacteria use oxygen as their terminal electron acceptor and in this case, the ΔE0' of the reaction is approximately 0.62 V. This still produces a -ΔG. The bottom line is that depending on the electron donor and acceptor that the organism uses, a little or a lot of energy (per electron) can be transferred and used by the cell as electrons are donated to the electron transport chain.
How do you calculate ∆E? It's pretty simple using the redox table- look for the final state (in V) of the electron (say, water, from reduction of O2) and subtract the initial state (say, as glucose). Using this chart your calculation ∆E would be 0.82 -(-0.43) = 1.25 volts. You do not need to worry (yet) about the number of electrons as ∆ volts have units of ∆ unit energy/unit charge (for example, Joules per Coulomb).
What are the complexes of the ETC?
ETCs are made up of a series (or at least one) of membrane associated redox proteins or (some are integral) protein complexes (complex = more than one protein arranged in a quaternary structure) that move electrons from a donor source, such as NADH, to a final terminal electron acceptor, such as oxygen. This particular donor/terminal acceptor pair is the primary one used in human mitochondria. Each electron transfer in the ETC requires a reduced substrate as an electron donor and an oxidized substrate as the electron acceptor. In most cases the electron acceptor is a member of the enzyme complex. Once the complex is reduced, the complex can serve as an electron donor for the next reaction.
How do ETC complexes transfer electrons?
As previously mentioned the ETC is composed of a series of protein complexes that undergo a series of linked redox reactions. These complexes are in fact multiprotein enzyme complexes referred to as oxidoreductases: the enzymes perform both oxidation (of the step in the chain "above" them, energetically) and reduction (of the complex in the complex in the chain below them) or reductases (again, in spite of the fact that the enzyme both oxidizes an electron donor and passes that electron to an electron acceptor). The one exception to this naming convention is the terminal complex in aerobic respiration that uses molecular oxygen as the terminal electron acceptor. That enzyme complex is referred to as an oxidase (even though it both oxidizes and reduces something). Redox reactions within these complexes are typically carried out by a non-protein component called a prosthetic group. This is true for all of the electron carriers with the exception of quinones, which are a class of lipids that can directly be reduced or oxidized by the oxidoreductases. In this case, both the Quinonered and the Quinoneox is soluble within the membrane and can move from complex to complex. The prosthetic groups are directly involved in the redox reactions being catalyzed by their associated oxidoreductases. In general these prosthetic groups can be divided into two general types: those that carry both electrons and protons and those that only carry electrons.
Question:
If the redox reaction is carried out by a non-proteinaecious "prosthetic group" (see list below)- why bother with the protein part of the complex?
The Electron and Proton carriers in the ETC
- Flavoproteins (Fp), these proteins contain an organic prosthetic group called a flavin, which is the actual moiety that undergoes the oxidation/reduction reaction. FADH2 is an example of a Fp.
- Quinones, are a family of lipids which means they are soluble within the membrane.
- NADH and NADPH are electron (2e-) and proton (2 H+) carriers, of course. The FADH2 we know from the citric acid cycle is actually bound to the enzyme succinate dehydrogenase.
- Cytochromes are proteins that contain a heme prosthetic group. The Heme is capable of carrying a single electron.
- Iron-Sulfur proteins contain a non-heme iron-sulfur clusters that can carry an electron. The prosthetic group is often abbreviated as Fe-S
Aerobic versus Anaerobic respiration
In the world we live in, most of the organisms we see around us breath air, which is approximately 20% oxygen. Oxygen is our terminal electron acceptor. We call this process respiration, specifically aerobic respiration. We breath in oxygen, it diffuses into our cells and to our mitochondria where it is used as the final acceptor of electrons from our electron transport chains. That is aerobic respiration: the process of using oxygen as a terminal electron acceptor in an electron transport chain.
While we may use oxygen as the terminal electron acceptor for our respiratory chains, the more general process of respiration evolved at time when oxygen was not a major component of the atmosphere. Respiration or oxidative phosphorylation does not require oxygen at all; it simply requires a compound with a high reduction potential to act as a terminal electron acceptor. Many organisms can use a variety of compounds including nitrate (NO3-), nitrite (NO2-), even iron (Fe+++) as terminal electron acceptors. When oxygen is NOT the terminal electron acceptor, the process is referred to as anaerobic respiration. The ability of organisms to vary their terminal electron acceptor provides metabolic flexibility and can ensure better survival if any given terminal acceptor is in limited supply. Think about this: in the absence of oxygen we die; but an organism that can use a different terminal electron acceptor can survive.
Question: What's the difference between anaerobic respiration and fermentation?
Here is a fun video in which a nice young man COMPLETELY FAILS TO UNDERSTAND what anaerobic respiration is, teaching us instead about fermentation. Beware of the internet! I'd say "you get what you pay for" but this is actually a site you have to pay for!
A generic example of a simple, 2 complex ETC
The figure below depicts a generic electron transport chain, composed of two integral membrane complexes; Complex Iox and Complex IIox. A reduced electron donor, designated DH (such as NADH or FADH2) reduces Complex Iox giving rise to the oxidized form D (such as NAD+ or FAD). Simultaneously, a prosthetic group within complex I is now reduced (accepts the electrons). In this example the redox reaction is exergonic and the free energy difference is coupled by the enzymes in Complex I to the endergonic translocation of a proton from one side of the membrane to the other. The net result is that one surface of the membrane becomes more negatively charged, due to an excess of hydroxyl ions (OH-) and the other side becomes positively charged due to an increase in protons on the other side. Complex Ired can now reduce the prosthetic group in Complex IIred while simultaneously becoming oxidized by Complex IIox. Electrons pass from Complex I to Complex II via thermodynamically spontaneous redox reactions, regenerating Complex Iox which can repeat the previous process. Complex IIred reduces A, the terminal electron acceptor to regenerate Complex IIox and create the reduced form of the terminal electron acceptor. In this case, Complex II can also translocate a proton during the process. If A is molecular oxygen, water (AH) will be produced. When A is oxygen the reaction scheme would be considered a model of an aerobic ETC. However, if A is nitrate, NO3- then Nitrite, NO2- is produced (AH) and this would be an example of an anaerobic ETC.
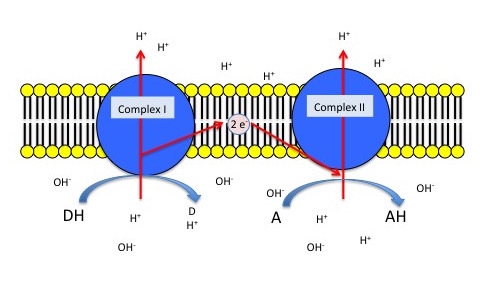
Generic 2 complex electron transport chain. In the figure, DH is the electron donor (donor reduced) and D is the donor oxidized. A is the oxidized terminal electron acceptor and AH is the final product, the reduced form of the acceptor. As DH is oxidized to D, protons are translocated across the membrane, leaving an excess of hydroxyl ions (negatively charged) on one side of the membrane and protons (positively charged) on the other side of the membrane. The same reaction occurs in Complex II as the terminal electron acceptor is reduced to AH.
Exercise 1
Thought question
Based on the figure above and using an electron tower, what is the difference in the electrical potential if (A) DH is NADH and A is O2 and (B) DH is NADH and A is NO3-. Which pairs (A or B) provide the most amount of usable energy?
In the nitrate vs nitrite example above, both the oxidized and the reduced forms have a charge of minus 1. So... how to you know which form is more oxidized?
The figure above has a problem- it depicts an electron floating within the membrane. This wouldn't happen- the membrane is not a "wire", it is an "insulator". You might draw your own corrected version.
Detailed look at aerobic respiration
The eukaryotic mitochondria has evolved a very efficient ETC. There are four complexes composed of multiple proteins and their prosthetic groups. These complexes labeled I through IV depicted in the figure below. The aggregation of these four complexes, together with associated mobile, accessory electron carriers, makes up the electron transport chain. This type of electron transport chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes.

The electron transport chain is a series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH2 to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane space, and oxygen is reduced to form water.
Complex I
To start, two electrons are carried to the first complex aboard NADH. This complex, labeled I, is composed of flavin mononucleotide (FMN) and an iron-sulfur (Fe-S)-containing protein. FMN, which is derived from vitamin B2, also called riboflavin, is one of several prosthetic groups or co-factors in the electron transport chain. These prosthetic groups (non-protein molecule required for the activity of a protein) are organic or inorganic non-peptide molecules bound to a protein, covalently or noncovalently; prosthetic groups include co-enzymes, which are the prosthetic groups of enzymes. The enzyme in complex I is NADH dehydrogenase (aka NADH Q reductase) and is a very large protein, containing 45 amino acid chains. Complex I can pump four hydrogen ions across the membrane from the matrix into the intermembrane space, and it is in this way that the hydrogen ion gradient is established and maintained between two aqueous compartments separated by the inner mitochondrial membrane.
Q and Complex II
Complex II directly receives FADH2, FADH2's electrons do not pass through complex I. The compound connecting the first and second complexes to the third is ubiquinone (Q). The Q molecule is lipid-soluble and freely moves through the hydrophobic core of the membrane. Once it is reduced, (QH2), ubiquinone delivers its electrons to the next complex in the electron transport chain. Q receives the electrons derived from NADH from complex I and the electrons derived from FADH2 from complex II, including succinate dehydrogenase. This enzyme and FADH2 form a small complex that delivers electrons directly to the electron transport chain, bypassing the first complex. Since these electrons bypass and thus do not energize the proton pump in the first complex, fewer ATP molecules are made from the FADH2 electrons. As we will see in the following section, the number of ATP molecules ultimately obtained is proportional to the number of protons pumped across the inner mitochondrial membrane.
Complex III
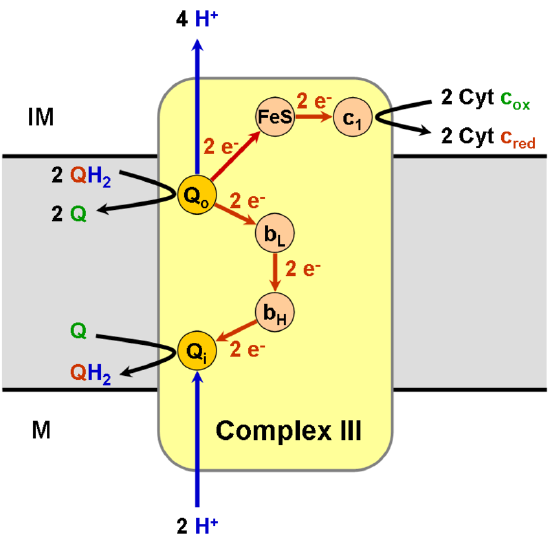
There's a lot of redox going on in this picture. Which is the upstream donor of electrons coming into Complex III? Which complex/molecule does Complex III reduce?
The third complex is composed of proteins that carry a number of prosthetic groups: a B-type cytochrome, a c-type cytochrome, and an Iron-sulfur cluster; this complex is called cytochrome bc1 complex. Cytochrome (= "cellular pigment") proteins have a prosthetic group that includes a metal bound by heme. The heme molecule is similar to the heme in hemoglobin, but it carries electrons, not oxygen. As a result, the iron ion at its core is reduced and oxidized as it passes the electrons, fluctuating between different oxidation states: Fe++ (reduced) and Fe+++ (oxidized). The heme molecules in the cytochromes have slightly different characteristics due to the effects of the different proteins binding them, giving slightly different characteristics to each complex. Complex III pumps protons through the membrane and passes its electrons to cytochrome c for transport to the fourth complex of proteins and enzymes (cytochrome c is the acceptor of electrons from Q; however, whereas Q carries pairs of electrons, cytochrome c can accept only one at a time).
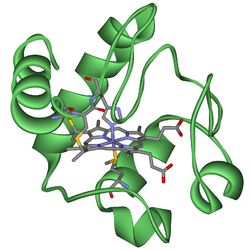
Cytochrome C
The cytochrome complex, or cyt c is a small hemeprotein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is an essential component of the electron transport chain, where it carries one electron. It is capable of undergoing oxidation and reduction as its iron atom converts between the ferrous and ferric forms, but does not bind oxygen. It transfers electrons between Complexes III (Coenzyme Q – Cyt C reductase) and IV (Cyt C oxidase).
Complex IV, cytochrome C oxidase
This complex contains two heme groups (one in each of the two cytochromes, a, and a3) and three copper ions (a pair of CuA and one CuB in cytochrome a3). The cytochromes hold an oxygen molecule very tightly between the iron and copper ions until the oxygen is completely reduced (the donor, cytochrome C, can only deliver one electron at a time). The reduced oxygen then picks up two hydrogen ions from the surrounding medium to make water (H2O). The removal of the hydrogen ions from the system contributes to the ion gradient used in the process of oxidative phosphorylation (as well as providing the -∆G required to drive other endergonic processes).
Here is a very nice video that includes the electron transport chain- I think it's very helpful, check it out! It starts with ATP synthase, which we haven't discussed yet, but that's fine, it'll explain why the cell is building that proton gradient!
I have one problem with this animation:There's really no discussion of how proton pumping works- the discussion's extremely vague- one might even come away with the notion that a gas forms within the matrix-domain of NADH dehydrogenase (complex I). In fact, the proton pump of complex I is entirely embedded within the membrane and isn't illustrated here at all. Presumably this is because the mechanism of pumping is a subject of debate. For the purposes of this class, you simply need to know- as mentioned in the video- that the endergonic formation of a proton gradient is powered by the exergonic redox reactions. In NADH dehyrogenase, this includes the oxidation of NADH by a flavin within the enzyme, some internal transfers of electrons within the enzyme, and then the transfer of the electron to a membrane-soluble quinone (coenzyme Q), to form a quinol. Each of these 2-electron transfers is illustrated below, with the oxidized molecules shown first.
Question:
Could NADH dehyrodrogenase reduce NAD+ as well as oxidize NADH? Under what circumstances might this happen?


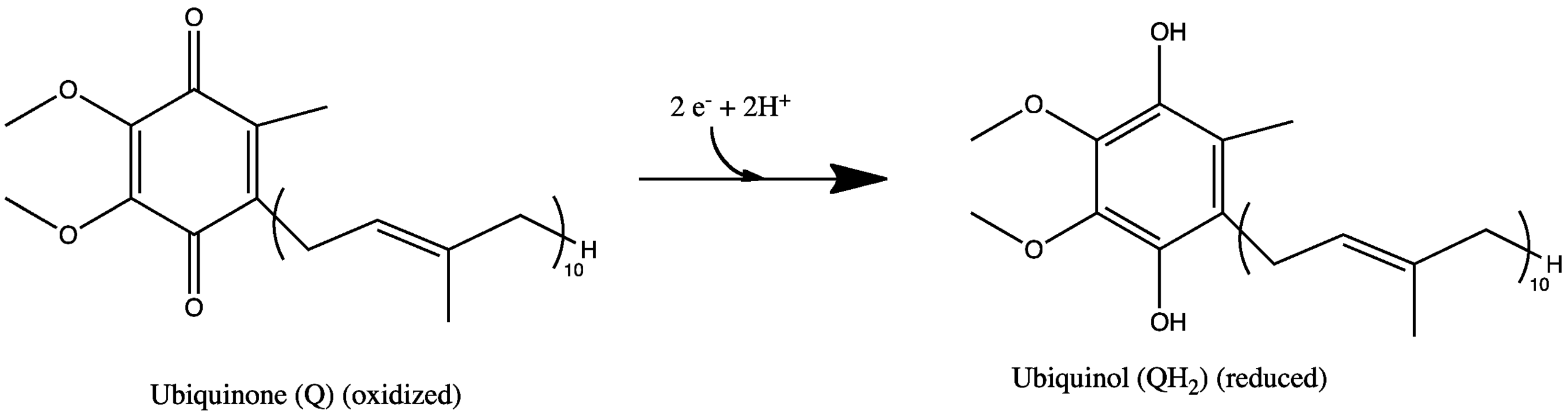
Chemiosmosis
The free energy from the series of redox reactions just described is used to pump hydrogen ions (protons) across the membrane, resulting in a concentration of protons on one side of the membrane. The uneven distribution of H+ ions across the membrane establishes both concentration and electrical gradients (thus, an "electrochemical" gradient), owing to the hydrogen ions’ positive charge and their aggregation on one side of the membrane.
If the membrane were open to diffusion by the hydrogen ions, the ions would tend to diffuse back across into the matrix, driven by their electrochemical gradient. Many ions cannot diffuse through the nonpolar regions of phospholipid membranes without the aid of ion channels. Similarly, hydrogen ions in the matrix space can only pass through the inner mitochondrial membrane via an integral membrane protein called ATP synthase (depicted below, and illustrated in the video recommended above). This complex acts as a tiny generator, turned by the protons moving down their electrochemical gradient. The movement of this molecular machine serves to both lower the activation energy of the ADP + Pi -> ATP reaction and couple the exergonic transfer of energy associated with the movement of protons down their electrochemical gradient to the endergonic addition of a phosphate to ADP, forming ATP.
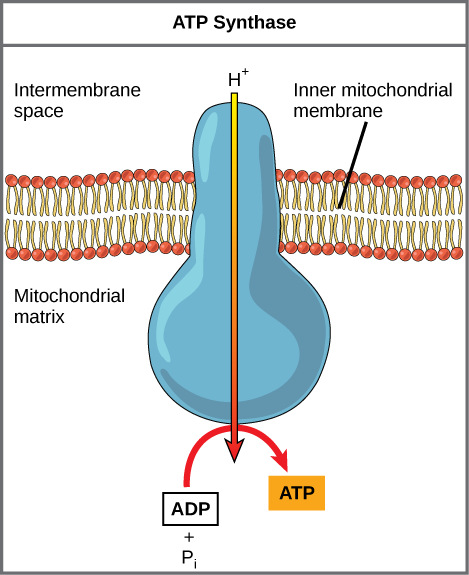
ATP synthase is a complex, molecular machine that uses a proton (H+) gradient to form ATP from ADP and inorganic phosphate (Pi). (Credit: modification of work by Klaus Hoffmeier). If you have had some O-chem and would like to dig a little deeper into the mechanism through which the mechanical flexing of this complex promotes ATP formation, check out this video.
Suggested Discussion
Dinitrophenol (DNP) is a small chemical that makes the membrane leaky to protons. It is sometimes used as a weight-loss drug, and can have lethal consequences. What effect would you expect DNP to have on the difference in pH across both sides of the inner mitochondrial membrane? Why do you think this might be an effective weight-loss drug? Why might it be dangerous?
In our cells, chemiosmosis is used to generate 90 percent of the ATP made during aerobic glucose catabolism; it is also the method used in the light reactions of photosynthesis to harness the energy of sunlight in the process of photophosphorylation (vs. oxidative phosphorylation via the ATPase of mitochondria). The overall result of these reactions is the production of ATP from the energy of the electrons removed from hydrogen atoms. These atoms were originally part of a glucose molecule. At the end of the pathway, the electrons are used to reduce an oxygen molecule to water.
Super-helpful video:
How ATP is made by ATP synthase

In oxidative phosphorylation, the pH gradient formed by the electron transport chain is used by ATP synthase to form ATP in a Gram- bacteria.
Suggested Discussion
Cyanide inhibits cytochrome C oxidase, a component of the electron transport chain. If cyanide poisoning occurs, would you expect the concentration of protons of the intermembrane space to increase or decrease? What effect would cyanide have on ATP synthesis?