1: The Basics of Climate Change
- Page ID
- 34456
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Search Fundamentals of Biochemistry
-
Historical Foundations of Climate Science:
- Explain the contributions of early scientists like Eunice Foote, John Tyndall, and James Hansen in establishing the link between CO₂ emissions and global warming.
- Discuss how political and economic interests have influenced public perceptions and policy on climate change.
-
Understanding the Greenhouse Effect:
- Describe Earth’s energy balance and how incoming solar radiation and outgoing infrared radiation maintain global temperature stability.
- Illustrate the role of greenhouse gases (CO₂, CH₄, N₂O) in absorbing infrared radiation and maintaining Earth’s warmth.
-
Quantitative Impact of Anthropogenic Emissions:
- Analyze how industrial activities have altered atmospheric CO₂ levels from pre-industrial values (~280 ppm) to current levels (427 ppm as of January 2025).
- Compare the magnitude of human-induced energy imbalances to familiar energy scales, such as atomic bomb detonations.
-
Global Warming Potential (GWP) and Gas Lifetimes:
- Calculate CO₂-equivalents using the GWP formula, and compare the relative contributions of CO₂, CH₄, and N₂O to global warming over a 100-year period.
- Explain why the long atmospheric lifetime of CO₂ results in cumulative warming effects, and methane’s higher short-term potency.
-
Interpreting Geological Climate Data:
- Utilize ice core and sediment data to interpret periodic changes in atmospheric CO₂ and temperature over the last 800,000 years.
- Evaluate how natural glacial-interglacial cycles compare to the current rapid increase in anthropogenic CO₂.
-
Orbital Forcing and Climate Feedback Mechanisms:
- Describe the Milankovitch cycles (eccentricity, obliquity, precession) and their role in triggering deglaciation.
- Assess the feedback loops involving CO₂ release from oceans and changes in dust deposition, and how these processes amplify climate change.
-
Linking Carbon Cycle Dynamics to Ocean Chemistry:
- Explain the chemical equilibria involving CO₂, H₂O, HCO₃⁻, and CO₃²⁻ and how they regulate ocean pH and atmospheric CO₂ levels.
- Discuss the role of weathering, ocean alkalinity, and primary production in modulating the carbon cycle.
-
Evaluating Climate Sensitivity Estimates:
- Differentiate between equilibrium climate sensitivity (ECS) and Earth Systems Sensitivity (ESS) in the context of doubling atmospheric CO₂.
- Interpret recent data suggesting that long-term warming (ESS) could be significantly higher than short-term estimates (ECS).
-
Critical Analysis of Climate Data and Models:
- Develop skills in evaluating and interpreting proxy data (e.g., ice cores, sediment records) used to reconstruct past climate conditions.
- Formulate arguments to address common climate change skepticism by comparing natural climate variability with unprecedented anthropogenic changes.
These learning goals provide a structured approach for biochemistry majors to understand the scientific principles underlying climate change, integrating knowledge from physics, chemistry, and biology to critically assess and address current and future environmental challenges.
prompt: Write a series of learning goals for the material below. The page is designed for junior and senior biochemistry majors.
Introduction
We've known for a long time that burning fossil fuels and releasing CO2 into the atmosphere would warm our climate. Perhaps the first paper addressing this, Circumstances affecting the Heat of the Sun's Rays, was published in 1856, before the US Civil War, by a woman scientist, Eunice Foote. John Tyndall (of the Tyndall effect) published more comprehensively on greenhouse gases in 1859. Given the complexity of the biosphere's climate, it was not until the 1980s that climate models became sophisticated enough for scientists like James Hansen to become convinced and alarmed enough to discuss in Congressional hearings the role of anthropogenic (made by humans) CO2 released into the atmosphere as a cause of ever-worsening global warming. The knowledge of human-induced climate change has been politicized and subjected to an orchestrated campaign of misinformation and disinformation by fossil fuel companies and their political contributors. We have delayed global actions for so long that we must act immediately and aggressively to address climate change before we reach climatic conditions that are so austere for humans that parts of the world become uninhabitable. Homo sapiens evolved in a world dominated by repetitive glaciation and deglaciation. Hans Joachim Schellnhuber, an atmospheric physicist, climatologist, and founding director of the Potsdam Institute for Climate Impact Research, has stated that humans have so affected the world that we have eliminated the possibility of the next glaciation cycle.
Many readers might not be familiar with the data and models supporting human-caused climate change. They may not be aware that climate scientists are almost unanimous in supporting climate data and models. As in any field, however, you will find outliers who don't and whose ideas carry disproportionate weight among climate change skeptics. Hence, we provide the basic data to show the relationship between increasing atmospheric CO2 and global warming, drought and flooding, ocean acidification, and biodiversity loss. We also provide supportive information, allowing users to address questions from those who question the reality of present human-induced climate change. We don't shy away from using basic physics as well, since most students studying biochemistry at the level found in this book have also studied physics and biology. In subsequent sections, we will address the biochemical consequences of climate change and how biochemistry can help mitigate it.
Greenhouse Effect
Before the advent of the Industrial Revolution, the Earth's climate was fairly constant since the last ice age, which peaked about 22,000 years ago (YA) and ended about 12,000 YA. There have been short (on a geological time scale) cooling periods since the end of the last ice age. Humans evolved around 200,000 years ago, with modern civilizations arising about 4000 BCE, so it could be said that humans are ice-age peoples (a distinctly Northern Hemisphere perspective). Humans have had the benefits of a fairly stable climate since then.
The sun's energy warms the Earth. If the Earth did not radiate back into space an equivalent amount of energy, it would slowly and continually warm. The Earth reflects energy in the form of light. In addition, as the Earth is heated by the sun, it releases heat in the form of infrared light (as do all warm objects). Earth's temperatures are stable when the sum of the energy emitted by the Earth equals the energy it receives from the sun. This is illustrated in Figure \(\PageIndex{1}\).
Figure \(\PageIndex{1}\): Energy balance for Earth as it adsorbs and emits solar radiation
Relatively constant levels of atmospheric CO2 have enabled our stable climate, a trace atmospheric gas, which has hovered around 280 parts per million (ppm) until the start of the Industrial Revolution in 1770. CO2 is a greenhouse gas, which, as anyone who has run an IR spectrum knows, absorbs in the infrared. CO2 in the atmosphere absorbs some of the infrared radiation released by the Earth, allowing the Earth to be warmer than in its absence. The CO2 effectively acts as an insulating blanket. In fact, without CO2 or other "greenhouse" gases, the Earth would be completely covered by snow.
Other greenhouse gases in the atmosphere include methane and nitrous oxide. The IR spectra of these gases are shown in Figure \(\PageIndex{2}\). Students who have taken organic chemistry labs and obtained IR spectra of samples always blank the instrument to remove spectral signals from both CO2 and H2O.
Figure \(\PageIndex{2}\): IR spectra of some greenhouse gases. NIST (ex: https://webbook.nist.gov/cgi/cbook.c...ndex=1#IR-SPEC)
Since the start of the Industrial Revolution, humans have been releasing ever-increasing amounts of CO2 into the atmosphere from burning fossil fuels and methane from agricultural practices and natural gas production. As of January 2025, CO2 levels have reached 427 ppm, while methane has increased to 1900 parts per billion (ppb) or 1.9 ppm.
Interactive graphs showing atmospheric CO2 and methane over time are shown below.
Increasing methane in the atmosphere contributes about 20% of the global warming effect of the more concentrated CO2, as it has an intense IR absorbance spectrum. It has a short half-life in the atmosphere (about 20 years) compared to CO2 (hundreds of years). Nitrous oxide (N2O) is also a powerful greenhouse gas that depletes ozone in the stratosphere. Our increased use of synthetic fertilizers and manure is the primary anthropogenic source of N2O. Its emission is accelerated from poorly drained farmlands.
The net effect is that the Earth is no longer in energy balance. The net contributions of energy input and output are expressed as energy/sec/m2 of the Earth's surface area. Hence, the units are Joule/s.m2 = Watts/m2, where 1 Watt is 1 J/s. From around 2005 to 2019, the average Earth Energy Imbalance (EEI) (or budget) was about +460 x 1012 W or 460 terawatts (TW) = 4.6 x 1014 W. Dividing this by the surface area of the planet (5.1 x 1014 m2), gives an average global value of +0.90 ± 0.15 W/m2 (or J/(m2s)). Newer and more refined estimates show it to be +1 W/m2, with most of the excess energy stored in the Earth's Oceans, which have a high specific heat capacity and cover 71% of Earth's surface area. Most of the remaining excess energy is used to warm land masses and melt ice shields and glaciers. 1 W/m2 might not seem like a lot but it is (pardon the pun), astronomically big.
The atomic bomb dropped on Hiroshima released about 1.8 x 1013 Joules (some estimates are as high as 9 x 1013 Joules) of energy. Assume for the sake of calculation that this energy was released in 1 second over the planet's entire surface (5.1 x 1014 m2). The earth energy imbalance for that one explosion was 0.035 J/m2.s (0.176 J/m2.s for the higher estimate). The ratios of the present imbalance (+1 W/m2) to the distributed bomb's imbalances are 1/0.035 = 28x for the lower energy estimate bomb or 1/0.176 = 5.7 for the higher energy estimate. Hence, the present imbalance, 1 J/m2s from human-caused climate change, is equivalent to detonating 6-28 Hiroshima-type atomic bombs every second. Other estimates are lower and closer to 600 bombs per day. Regardless, these values should give you a dramatic sense of the imbalance and how unsustainable it is.
Figure \(\PageIndex{3}\) below represents the Earth's energy budget.
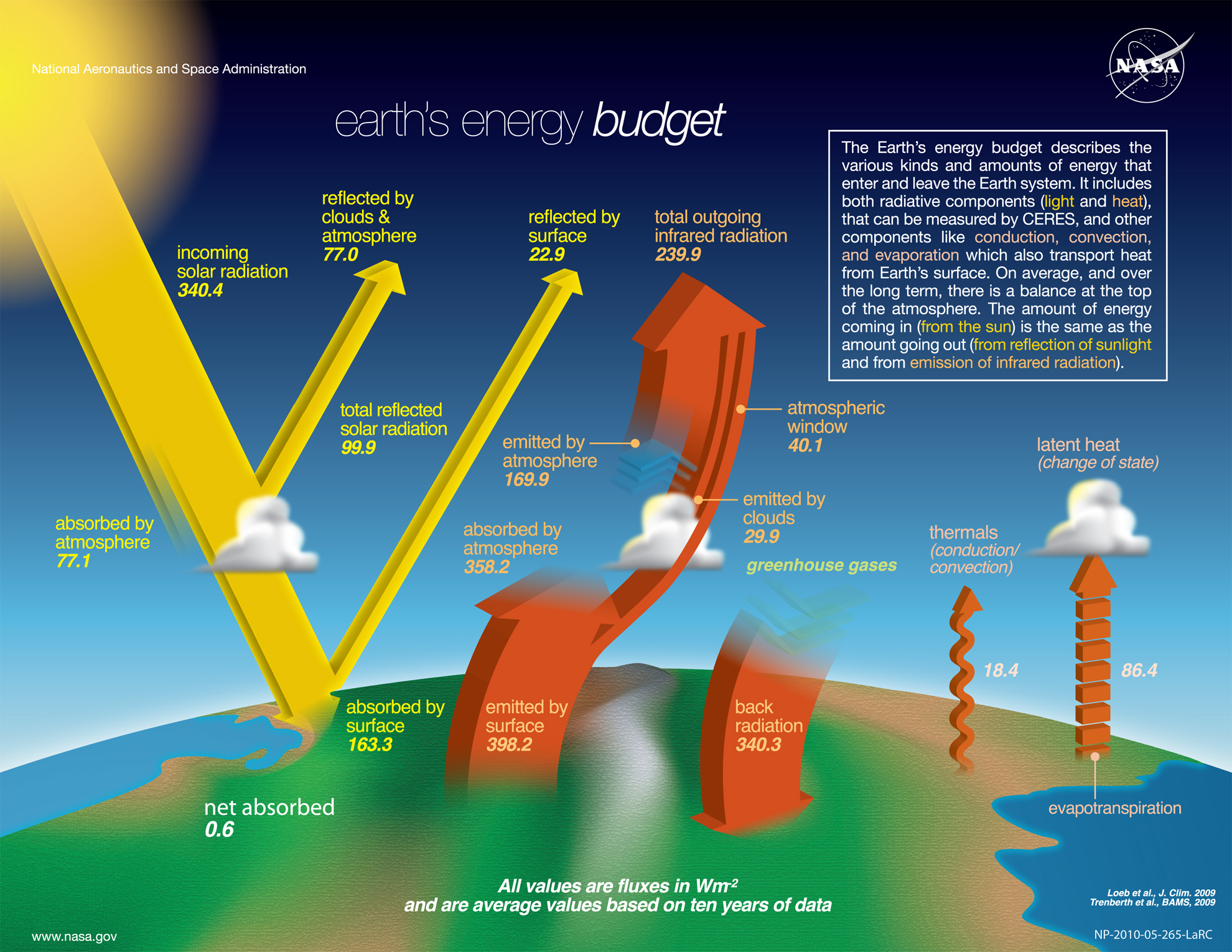
Figure \(\PageIndex{3}\): Earth's energy budget, with incoming and outgoing radiation (Values are shown in W/m2). Satellite instruments (CERES) measure the reflected solar and emitted infrared radiation fluxes. Year 2021 update: Net absorbed energy (shown as 0.6) rose to above 1.0 W/m2 based on independent CERES and ocean heat content measurements. (see Fig. 1 in Loeb et al.(2021), Geoph. Res. Let 48 (13), doi:10.1029/2021GL093047). Public Domain
The global warming potential (GWP) calculates the total contribution of all emitted greenhouse gases. It is expressed in units of CO2 equivalents. It adds the contribution of other greenhouse gases like CH4 and nitrous oxide (N2O), each with unique IR absorption spectra (as shown in Figure 2 above) and atmospheric half-lives. The IPCC uses a 100-year time frame for the calculation of the GWP, which is often abbreviated as GWP100, and uses this formula:
\begin{equation}
\mathrm{CO}_2 \text { equivalent } \mathrm{kg}=\mathrm{CO}_2 \mathrm{~kg}+\left(\mathrm{CH}_4 \mathrm{~kg} \times 28\right)+\left(\mathrm{N}_2 \mathrm{O} k g \times 265\right)
\end{equation}
- CO2 has a GWP of 1 by definition since it is the reference. Its time frame in the atmosphere (100s to 1000 years) doesn't matter since it is the reference.
- CH4 has a GWP of around 27-30 over 100 years, reflecting its higher IR absorbance but lower lifetime (around 12 years).
- N2O has a GWP of around 265-273 over a 100-year timescale and a lifetime of around 109 years.
Water is also a greenhouse gas, as you can attest to on humid days, and its lack in the atmosphere in deserts leads to a large temperature drop at night. It's very different than other greenhouse gases. Its concentration varies enormously (from 40 ppm to 40000 or more) based on humidity and precipitation events, which remove it from the atmosphere. The amount of water in the atmosphere increases with increasing global temperatures, which gives rise to more intense precipitation events and warmer temperatures in a positive feedback loop. Its concentration in the atmosphere changes enormously on the time scale of hours and days, so its half-life in the atmosphere is short. In contrast, the half-life of CO2 in the atmosphere is measured in decades to centuries.
The basis for methane's contribution to climate change needs a clearer explanation. Given its IR absorption spectra, it is a very potent greenhouse gas. Many oil companies have indicated a commitment to decreasing methane leakage from pipelines, which would be helpful. Still, these efforts may draw attention away from the fact that CO2 is the biggest problem. Over 20 years (not 100 as in the GWP calculations above), methane is 80 times as potent as CO2. Those numbers sound ominous (80X and 20 years, which is not long away)! But you shouldn't compare the two on a kg/kg basis, as the warming from CO2 could last 1000 years, but from methane only 20 years, after which it disappears. Given its short lifetime in the atmosphere, the long-term climate effect of methane depends on the rate (megatons/year) at which it is added to the atmosphere. Still, for CO2, it is the net mass added over a longer time, which has a cumulative effect. CO2 effects continue to increase, but methane effects wane quickly. So you can't easily compare warming over the first 20 years. Consequently, a delay in acting on methane is minimal compared to a delay in acting on CO2.
Methane contributes to about 30% of net warming, and about 60% of the methane is probably from human activities. As we hit the IPPC Paris target of 1.5 0C, about 30% of that, or about 0.45 0C, is from methane; hence, 0.27 0C comes from human sources. Decreasing human sources of methane will help, but not if we keep using our efforts to remove methane as a "political" way not to increase our push for net-zero emissions of CO2.
Climate changes over the last million years
Climate has always changed. Our present period is no different, so action is unnecessary.
Indeed, the Earth has been subject to cycles of glaciation and deglaciation for hundreds of thousands of years. Luckily, we can determine atmospheric levels of CO2 dating back hundreds of thousands of years by measuring entrapped CO2 in ice cores from Antarctica and Greenland. In addition, we've been able to infer the temperature over this time frame using proxies for temperature (tree rings, fossils, and more, as described in section 31.2). Figure \(\PageIndex{4}\) shows how atmospheric CO2 and temperature have varied over the last 800,000 years using ice core data.
Several key features of the graph should be apparent:
- Both atmospheric CO2 and temperature change (ΔT) are periodic. So yes, it is true that "climate changes," as climate change skeptics argue.
- Both CO2 and ΔT change in synchrony. An obvious question might be what changes first. Does ΔT drive CO2 changes or vice versa? More on that in a bit.
- The CO2 levels in more modern times (right-hand side of the graph) have soared in ways not seen in the last 800,000 years! CO2 emissions from the burning of fossil fuels cause this change.
In those 800K years, the Earth has experienced cycles of glaciation/deglaciation with recurring ice ages. Figure (\PageIndex{5}\) below shows a depiction of the last ice age, which peaked 21,000 years ago (left). At that time, the ice cap over New York City was about 1 mile high (right) as CO2 was 185 ppm!
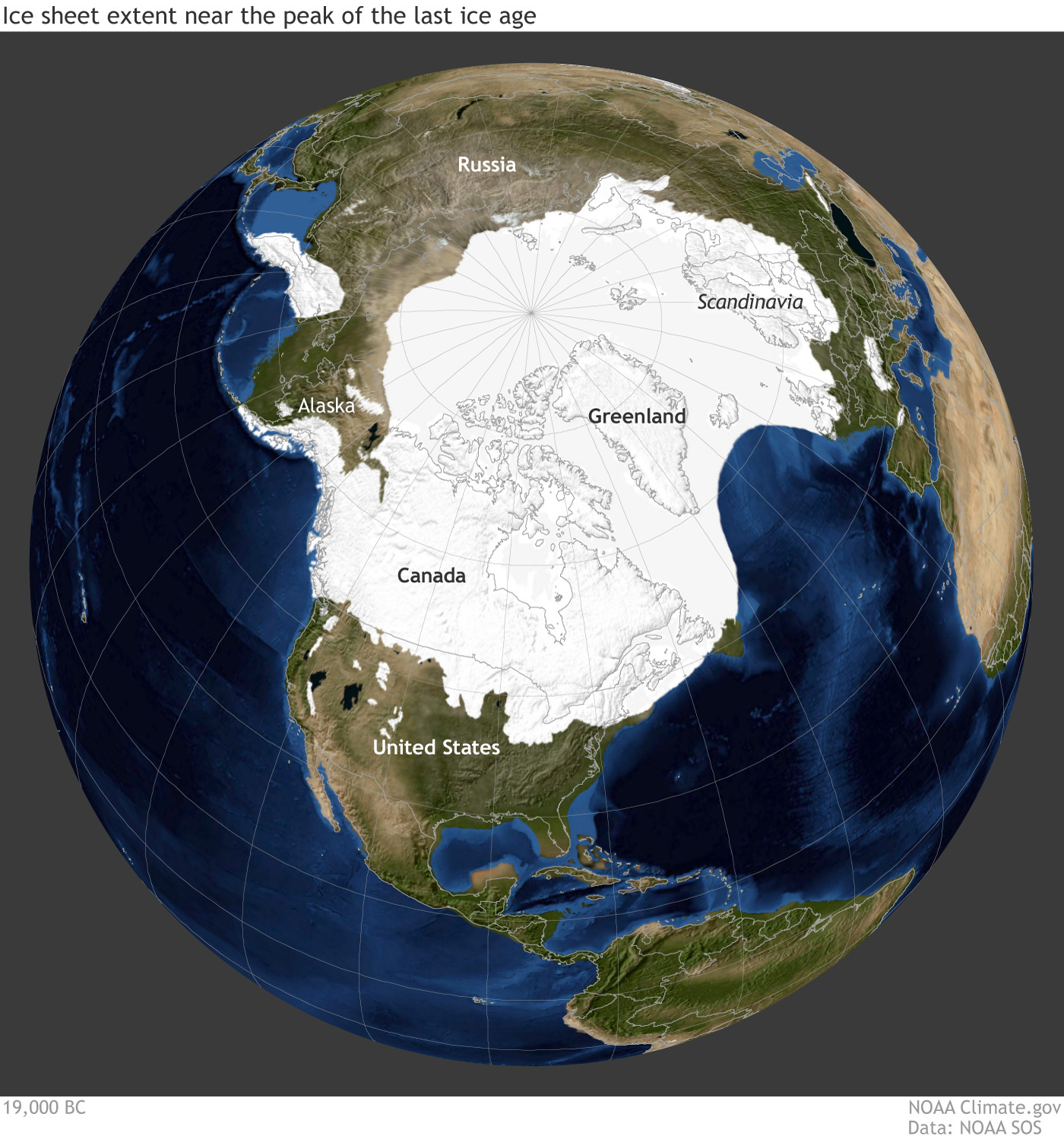 |
By 5000 BCE, the glacier had retreated to more modern levels, leaving ice over the Arctic Ocean and Greenland. CO2 levels were then around 260 ppm. A change of just 100 ppm in CO2 was sufficient to melt the Northern Hemisphere glaciers. The image above is not "Northern Hemisphere-Centric" since the great glaciers were localized in the Northern Hemisphere in the ice ages. That's because glaciers grow over land, and most of the land on the planet is in the Northern Hemisphere. (Our climate studies won't include when one continent - Pangea- existed.) The video below shows an animation of the Northern Hemisphere ice shield as it changed from 19,000 BCE to now to a projected future that assumes little action to change CO2 emissions. Pay special attention to the graphs, which also show sea level changes.
Best estimates by Tierney et al now show that during the last ice age, the average global temperature was 6 degrees Celsius (11 F) cooler than today, which in the 20th century is 14 C (57 F). The Arctic, however, was much colder (about 14 C or 25 F). The group also came up with an estimate of climate sensitivity, the increase in temperature with increasing CO2. That value is a rise of 3.4 °C (6.1 °F) for a doubling in CO2. In 1896, Arrhenius, recognizing that CO2 was a greenhouse gas, calculated that doubling atmospheric CO2 would cause a rise of 4-5 °C. No one can say we haven't known!
A new value for climate sensitivity, giving a rise of 4.8 °C (8.6 °F) for a doubling of CO2, was determined by Hansen et al (2023) using more precise data about temperatures and CO2 concentrations derived from isotopic analyses of ice core samples. If this value holds, the environment needed for human life and our present society is seriously jeopardized unless we take immediate measures to stop emissions and cool the planet. In addition, the rate of increase in global and ocean temperatures has accelerated after 2010, and the likely culprit is an actual decrease in toxic SO2 aerosols released by ocean vessels in the North Pacific and Atlantic Oceans and China from the reduction of dirty coal-burning facilities. Aerosols reflect incident solar light and increase cloud formation, both of which help cool the planet. Paradoxically, reducing anthropogenic aerosols, while great for cardiovascular and pulmonary health, will accelerate global warming. India would be much warmer now if not for the high aerosol concentrations from smog that covers many of its large cities and countryside.
Climate, CO2, and temperature have always changed over geological time, but our present rise in anthropogenic CO2 in such a brief time is unprecedented. This has led to CO2 levels far exceeding those during the warmer interglacial periods when Northern Hemisphere glaciers retreated.
The Ice Ages, CO2, and Temperature
It's not increasing CO2 that is causing any observed increases in temperature. CO2 is going up after temperature increases, so we don't have to worry about CO2 levels. It's a natural process requiring no action to reduce fossil fuel use. Why reduce it if it doesn't cause global warming?
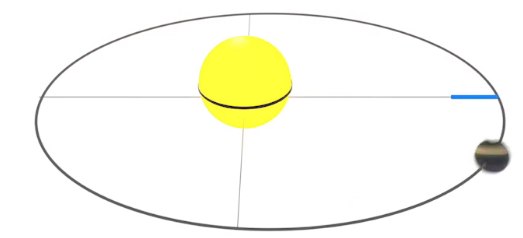
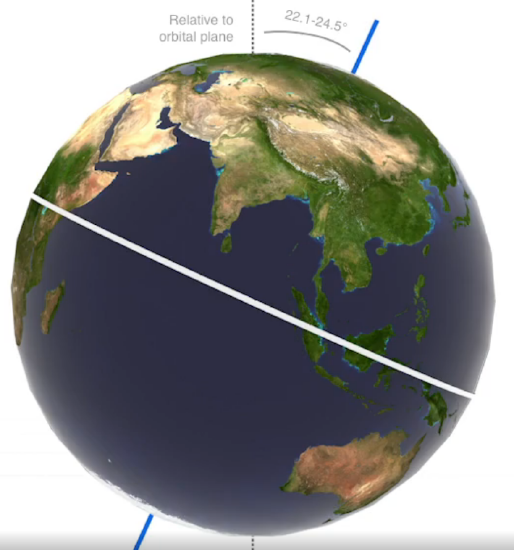
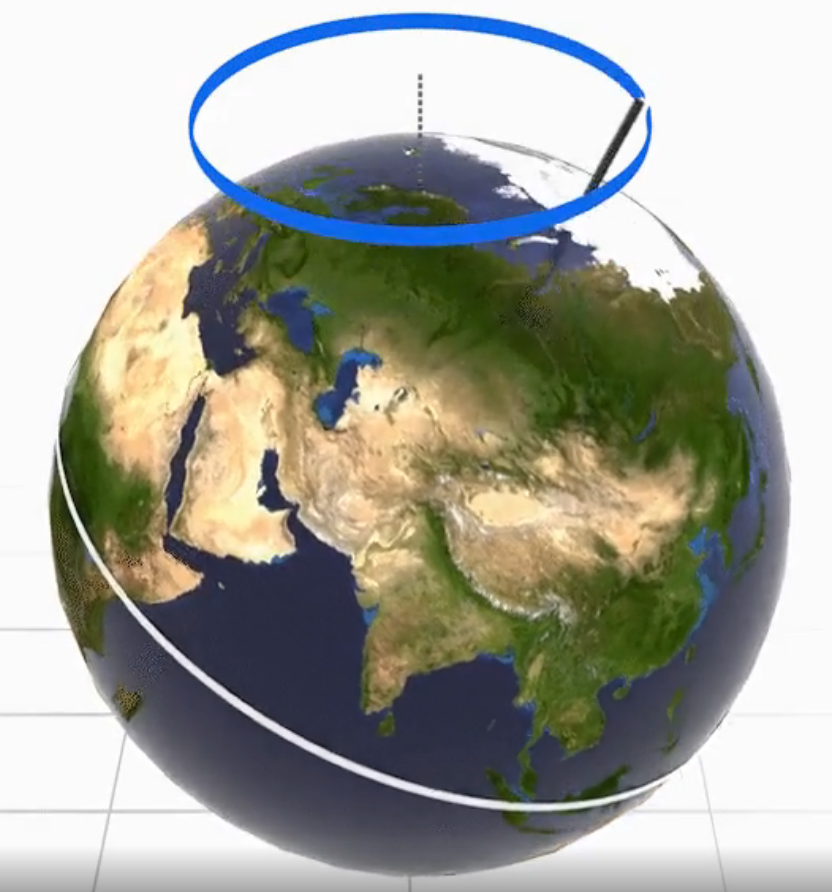
Data and models show that the global increase in temperature is driven mainly by increases in CO2 (and not increasing temperatures driving increasing CO2) as the predominant cause. That begs the question as to what starts the process of deglaciation. It turns out that cyclic increases in solar irradiance that increase temperatures, especially in the Northern Hemisphere, start deglaciation. A prime factor is the changes in the orbital dynamics of the Earth around the Sun. As you know, the orientation of the Earth's rotation axis remains generally fixed and pointed in the same orientation as the Earth rotates around the sun. This fixed orientation leads to Earth's annual spring, summer, fall, and winter cycles. In the winter, the northern hemisphere is pointed away from the sun, leading to decreased solar irradiance per square meter in the Northern Hemisphere, causing winter there. When the Earth is on the opposite side of the sun, the axis points in the same direction but tilts towards the sun, leading to summer in the northern hemisphere. However, the orbital dynamics of the Earth do change cyclically over long periods. These long-term changes in the Earth's orbital shape (eccentricity), tilt (obliquity), and wobble (precession) are called the Milankovitch Cycle and are illustrated in Figure \(\PageIndex{6}\). These cycles cause small temperature increases that start deglaciation. Click on each image below to download and view short videos illustrating these orbital changes.
Change in eccentricity (orbital shape) - (100,000 yr cycle)
Change in obliquity (tilt) (41,000 yr cycle)
Axle precession (wobble) (26,000 yr cycle)
Figure \(\PageIndex{6}\): The Milankovitch cycle showing changes in the Earth's orbital dynamics with respect to the sun. https://climate.nasa.gov/news/2948/m...arths-climate/
Based on these cycles, Milankovitch calculated that recurring ice ages should occur approximately every 41000 years. Ice ages did occur at this interval from about 3 million years ago (MYA) to 1 million years ago (MYA). About 800,000 years ago, they lengthened to about 100,000 years, corresponding to the Earth's eccentricity cycle. The increased cycle duration led to longer-lasting glaciers that moved further south into the Northern Hemisphere. One likely explanation for the increase in time between ice ages is that repeated glaciation/deglaciation eroded the bedrock in the Northern Hemisphere, converting it to regolith (rocks, soil, and dust). This allowed an increased velocity of movement of the glaciers to the south due to decreased frictional resistance and thicker ice cap formation (more time to accrue ice), which required a longer time to melt. This also provided a positive feedback loop as the increased northern ice area would reflect more of the sun's energy into space, cooling the planet. Punctuating these rhythmic orbital and ice age cycles are other events, such as large volcanic eruptions, asteroid impacts, etc, that could produce minor to major changes in climate and resulting mass extinctions.
Figure \(\PageIndex{7}\) shows how a combination of tilt angle, precession axis, and orbital shape at around 200 KYA (narrow rectangle across all the graphs) combined to lead to low glacial ice volume (bottom graph).
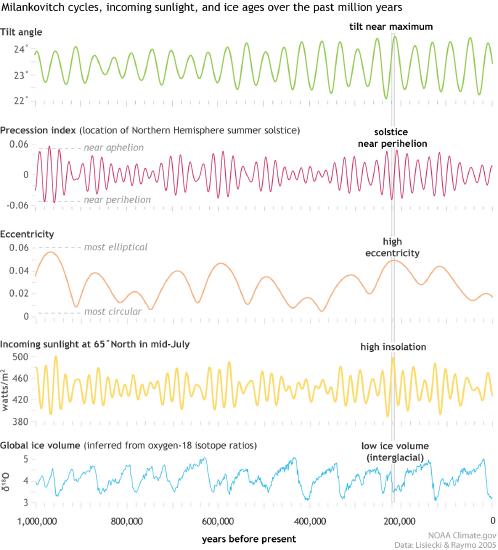
Figure \(\PageIndex{7}\): Milankovitch cycle contribution to ice volume over the past 1M years
If orbital changes (or forcing) trigger deglaciation, what is the role of increasing levels of the greenhouse gas CO2, which covary with temperature (see Figure 3)? Temperature increases derived from orbital and hence solar "forcing" seem to precede CO2 increases for short periods (perhaps 100 - 200 years). After that, CO2 causes almost all global temperature increases during deglaciation, with CO2 and temperature increasing together. A global increase of about 0.3 °C due to the Milankovitch cycle leads to greater Northern Hemisphere irradiance. This causes localized and limited melting of the Northern ice shield, leading to increased ocean temperatures in the northern oceans. These increases slow a major ocean current (the Atlantic Meridional Overturning Circulation - AMOC), inhibiting the burial and return of cold water in tropical and southern oceans. This, in turn, led to a warming in the south, accompanied by the release of large amounts of CO2 stored in the oceans (see Carbon Cycle in 31.3). The release of this greenhouse gas was then responsible for most of the warming that led to massive degradation. This "interhemispheric see-saw" heat transfer from northern to southern waters is key. For most of the warming during glacial melting, CO2 and temperature change synchronously.
Interpreting climate data is difficult. For example, it was found through measuring 15N/14N ratios that gases like N2 and, by extension, CO2 could rapidly diffuse through the compacting snow (firn, comprising the top 50-100 meters of the ice cap) until it became trapped in the solid ice beneath it. This would lead to "newer" CO2 in older ice samples, and the conclusion that temperature changes preceded changes in CO2. Corrections are made to the data to address the "apparent" time shift.
The CO2 trapped in bubbles in the ice core samples from Antarctica reflects global CO2 levels given the atmospheric circulation. However, the temperatures measured from the same core samples (see Chapter 31.2) represent local (Antarctic) temperatures. Ice core samples from Greenland and ocean sediment samples from around the world determine temperatures at different locations over time. All of these data are required to model the climate. Combined, they lead us to our present interpretation of the linkage of CO2 and temperature rise over time.
Increased solar irradiance on Earth, resulting from cyclic changes in the Earth's orbit, leads to short, small temperature increases in the Northern Hemisphere. These increases release the greenhouse gas CO2 from the oceans, causing synchronous warming of the planet and subsequent deglaciation.
So when skeptics say that temperature increases precede CO2 increases, you can acknowledge they did but that the bulk of the warming is attributed to increasing CO2 released from ocean stores, leading to synchronous temperature increases and deglaciation. Using the words of chemistry, small temperature increases from orbital forcing catalyzed the release of huge amounts of CO2 dissolved in the ocean. Chapter 31.3 will explore the carbon cycle in more detail and examine how it affects CO2 levels.
Termination of the Ice Ages
How did the ice ages terminate? Contributions from the orbital forcing derived from the Milankovitch cycle play a part. Another factor seems to be dust derived from regolith, which is made by glacier movement, as mentioned above. How can that hypothesis be tested? Using proxies for dust, namely iron and long-chain n-alkanes (derived from plant waxes) that have been deposited in sediments. First, look at a graph of CO2 and temperature changes and superimpose those on iron and long-chain fatty acid levels, as shown in Figure \(\PageIndex{8}\).
| |
A close examination of the two vertically aligned graphs from around 120 K to 130 KYA shows that iron and n-alkane depositions are at a minimum while CO2 and temperature are peaking! What explains this negative correlation? It depends on the intimate connection of the biosphere with the nonbiological world (an arbitrary distinction).
Iron and n-alkanes are circulated and delivered in dust. The long-chain alkanes, highly abundant in waxes and enriched in odd carbon number chains, were presumably derived from leaf waxes, which prevent water loss from plants, especially during higher temperatures. Dust deposits were first observed in geological time in the switch from the warmer Pliocene (5.3 to 2.6 MYA) to the Pleistocene (2.6 MYA to 11.7 KYA, see Figure 8 below). During the warmer Pliocene, the difference in global and atmospheric temperatures was lower, and with this smaller temperature gradient, winds that could globally transfer dust would be diminished. Also, the warmer Pliocene (5.3 to 2.6 MYA) would have had more rain, which would have removed dust from the global circulation.
As temperatures cooled in the Pleistocene (2.6 MYA to 11.7KYA), cycles of glaciation would produce more dust-containing regolith (rocks, soil, and dust), which would be dispersed through stronger global winds from higher temperature gradients and less rain. Dust contains carbon (for example, long-chain fatty acids) and, perhaps more importantly, iron, which is needed for oceanic phytoplankton growth. Without Fe, the uptake of CO2 by phytoplankton (primary production) would not occur, leading to increased CO2 in the atmosphere. Stronger regional atmospheric winds would lead to increased upwelling of nutrients and deep ocean CO2. The CO2 would enter the atmosphere more readily in the absence of dust deposition of iron.
In summary:
- High CO2 and high temperature (lower global temperature gradients, more rain) are associated with low dust, as measured with the proxies Fe and n-alkanes. Low dust leads to low deposition of Fe and n-alkanes in the ocean, which decreases phytoplankton primary production and the fixation of CO2 into biomass. This leads to increased CO2 movement from the ocean to the atmosphere, increasing temperature. This is an example of a positive feedback loop (higher temperatures leading to higher temperatures).
- Low CO2 and low temperature (higher global temperature gradients, stronger winds, less rain) are associated with high dust with Fe and n-alkanes deposition. This increases phytoplankton primary production and decreases CO2 movement from the ocean to the atmosphere in a negative feedback loop.
By the end of a glacial deposition cycle, dust, blown by stronger winds from higher temperature gradients, was increasingly deposited on the ice sheets. Along with leading to more heat absorption by the sheets, it would also decrease their reflectivity (albedo). Both effects would promote ice sheet melting. Also, a cooler planet during the glacial maximum had less precipitation, which, along with lower CO2, would lead to more plant and tree death, increasing soil erosion and desertification, both effects which would have increased dust production and its deposition on ice sheets. Then, when CO2 rose to 280 ppm, plant life renewed, and dust levels dropped.
Climate change from 66 million years ago to now
Antarctic ice core data cover the last 2 million (M) years. Ocean sediment data can go back to 66 million years ago (MYA), just before the dinosaurs died after the massive asteroid impact formed the Chicxulub crater buried underneath the Yucatán Peninsula in Mexico. A brief review of geological eras, periods, and epochs is shown below in Figure \(\PageIndex{9}\)
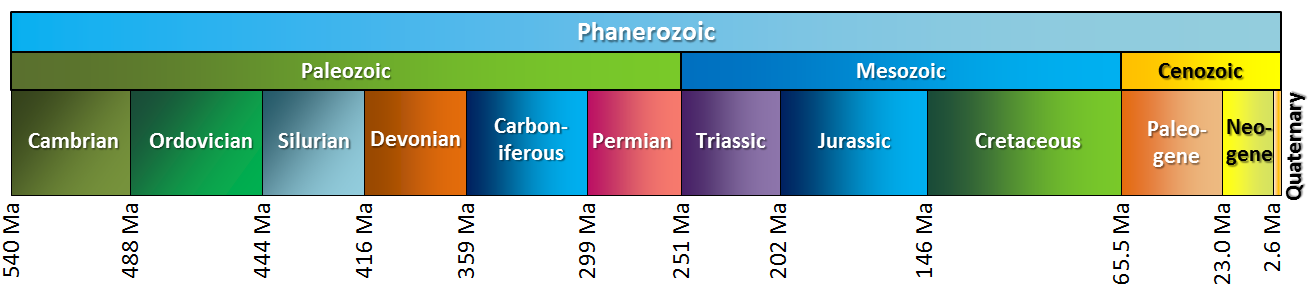
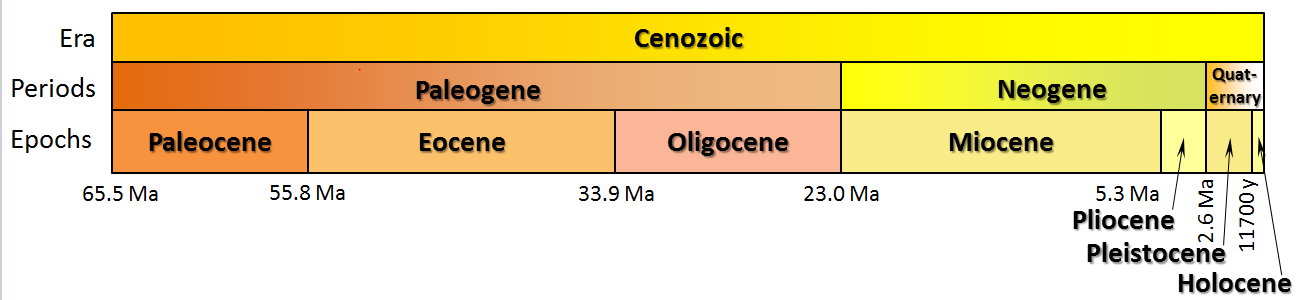
Figure \(\PageIndex{9}\): Geological Era, Periods and Epochs
CO2 levels and associated temperatures derived from ocean sediment cores going back to 66 MYA are shown in Figure \(\PageIndex{10}\).
Figure \(\PageIndex{10}\) CO2 levels (red) and temperatures (blue) derived from ocean sediment cores going back to 66 MYA = 66,000 KYA . Data from Rae et al. Annual Review of Earth and Planetary Sciences, 49, 2021
Again, note the parallel rise and fall of CO2 and temperature. Eventually, they fell further in the Pliocene (5.3 to 2.6 MYA) and Pleistocene (2.6 MYA to 11.7KYA) epochs with cyclic glacier/interglacial periods we've discussed above. Northern hemisphere glaciation started in the late Miocene (10 to 6 MYA), and both poles of the planet had glacial sheets.
The time frame shown in Figure 9 encompasses the Cenozoic era (65 MYA when the dinosaurs died to about now). CO2 levels were much higher than today in the greenhouse Paleocene and Eocene eras, but decreased to about 500 ppm in the Oligocene (34 MYA). An almost stepwise drop in CO2 and temperature occurred in the Eocene to Oligocene transition (EOT), about 33 MYA. Data shows the development of large ice sheets in Antarctica at that time. Before the EOT (33 MYA), Antarctica was ice-free, as shown in the recreation in Figure \(\PageIndex{11}\).

Figure \(\PageIndex{11}\): Reconstruction of the West Antarctic mid-Cretaceous temperate rainforest. Image credit: J. McKay / Alfred-Wegener-Institut / CC-BY 4.0. https://www.sci.news/othersciences/p...ing-09921.html
Proxy temperature data show that the transition was most likely caused by decreased CO2, and some orbital forcing was probably involved. Present models still struggle to explain the EOT (33 MYA) transition, but it is clear that both CO2 and temperature decreased. Where did the CO2 go? Most assuredly into the oceans.
To understand that, we have to understand a bit about the carbon cycle, which we will discuss more fully in the next chapter section. Let's briefly discuss the role of atmospheric CO2 and its interaction with the ocean. The main gases in the atmosphere, N2 and O2, are found in very low concentrations in the ocean since they are nonpolar and generally unreactive. CO2 is also a nonpolar trace gas, but in contrast, it can readily react with water to form HCO3- and CO3-2, which are abundant in ocean reserves. Hence, the ocean chemistry of CO2 determines, in large part, the levels of atmospheric CO2. The coupled reactions of CO2 are shown below.
\begin{equation}
\mathrm{CO}_2(\mathrm{~g}, \mathrm{~atm}) \leftrightarrow \mathrm{CO}_2(\mathrm{aq}, \text { ocean) }
\end{equation}
\begin{equation}
\mathrm{CO}_2(\mathrm{aq} \text {, ocean })+\mathrm{H}_2 \mathrm{O}(\mathrm{I} \text {, ocean }) \leftrightarrow \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+\mathrm{HCO}_3^{-}(\mathrm{aq})
\end{equation}
\begin{equation}
\mathrm{H}_2 \mathrm{O}(\mathrm{I})+\mathrm{HCO}_3^{-}(\mathrm{aq}) \leftrightarrow \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+\mathrm{CO}_3{ }^{2-}(\mathrm{aq} \text {, sparingly soluble })
\end{equation}
This chemistry helps determine the pH of the ocean. Figure \(\PageIndex{12}\) shows atmospheric CO2 and ocean pH levels over the last 66 million years.
V3GraphCO2_pH.png?revision=1&size=bestfit&width=963&height=577)
Figure \(\PageIndex{12}\): Atmospheric levels of CO2 and ocean pH over the last 66 million years
Before the EOT at 34 MYA, atmospheric CO2 levels were higher and ocean pH levels lower (around 7.7). After the EOT (33 MYA), atmospheric CO2 is much lower, and ocean pH is higher (more basic, 7.9, rising to 8.1). What happened to the CO2 is a bit unclear. Atmospheric CO2 decreased by moving into the oceans, but wouldn't that have lowered the pH based on the chemical equations presented above? It would have, but it turns out that the ocean alkalinity is determined not just by H3O+ produced by the equations above but by the dissolved inorganic carbon ions, HCO3- (aq) and CO32- (aq), which are conjugate bases. Increased HCO3- (aq) and SiO4-2 (aq) from weathering solid carbonates and silicates that entered the oceans would raise the pH of the oceans.
A little review of introductory chemistry helps here.
Let's take bicarbonate, the weak conjugate base of the weak acid carbonic acid. HCO3- can act as both an acid and base.
Rx 1: Acts as an acid: HCO3- (aq) + H2O (l) ↔ H3O+(aq) + CO32- (aq)
\begin{equation}
K_{a 2}=\frac{\left[\mathrm{H}_3 \mathrm{O}^{+}\right]\left[\mathrm{CO}_3^{2-}\right]}{\left[\mathrm{HCO}_3^{-}\right]}=4.7 \times 10^{-11}
\end{equation}
Rx 2: Acts as a base: HCO3- (aq) + H2O (l) ↔ H2CO3 (aq) + OH- (aq)
\begin{equation}
K_{b 2}=\frac{\left[\mathrm{H}_2 \mathrm{CO}_3\right]\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{HCO}_3^{-}\right]}=2.2 \times 10^{-8}
\end{equation}
The equilibrium constant for the reaction of HCO3- as a base is much larger, so bicarbonate is a stronger base than acid.
Whatever the mechanism of the CO2 drawdown, it led to decreasing temperatures in the EOT transition. The ocean's increased alkalinity also consumed H3O+, increasing ocean pH.
Figure \(\PageIndex{13}\) summarizes planetary temperatures across geological time.
Figure \(\PageIndex{13}\): Earth's temperature over 500 million years. https://commons.wikimedia.org/wiki/F...alaeotemps.png. (Excel available). Creative Commons Attribution-Share Alike 3.0 Unported
Several key features should be noted. The last time CO2 was as high as today (415 ppm) was about 3 million years ago. Repetitive cycles of glaciation/deglaciation are obvious in the Pleistocene (2.6 MYA to 11.7 KYA).
At around 56 MYA, a temperature spike of about 50 - 9 0F (or even more) on an already warmer planet occurred over about a 100 K-year timeframe. This is known as the Paleocene/Eocene thermal maximum (PETM). It is the closest example to the present rate of CO2 injection into the atmosphere so it gives us some basis to ascertain what we may seen with our present spike in CO2. The poles warmed to nearly 70 0F and alligators and palm trees were found there. The warming also led to the spread of tropic rainforests from the equator, allowing the evolution and proliferation of new plant species including flowering plants and an incredible biodiversity of insects, bird,s and animals that relied on them. Flowering plants produce fruits, which helped drive the evolution of mammals and the first true primates, including the tiny Teilhardina magnoliana. It may have resembled the picture below. (https://en.wikipedia.org/wiki/Teilhardina). Big eyes and hands would allow them to find fruit in the tropical forests.
The temperature increase was caused by a dramatic spike in CO2 from deep-sea volcanoes and vents, which also led to a dramatic drop in ocean pH as measured by the loss of deep-sea CaCO3 (chalk). Methane hydrates were also released. These environmental and biosphere changes are visually evident in geological deep-sea sediment records as shown in Figure \(\PageIndex{14}\).
Figure \(\PageIndex{14}\): Overview of the Paleocene–Eocene Thermal Maximum (PETM, 55.5 MYA) data from deep-sea records and the terrestrial Polecat Bench
(PCB) drill core against age. Westerhold et al. Clim. Past, 14, 303–319, 2018. https://doi.org/10.5194/cp-14-303-2018. Creative Commons Attribution 3.0 License
Sediment cores were taken at various sites (1262, 1267, 1266, 1265, 1263, and 690) aligned from left to right according to the water depth from deep to shallow. Note that 55.93 million years ago, at the start of the PETM, there was a sharp transition from light brown/gray, enriched in chalk, to dark brown, enriched in clay. Ocean acidification dissolved the chalk. It took over 100,000 years to recover.
This very short time frame is called the Paleocene/Eocene thermal maximum (PETM,55.5 MYA), which shows very quick spikes (on the geological time scale) can and do occur. Approximately 1.5 petagrams (1015) of CO2 were released annually during the PETM. Now, we are releasing about 25 petagrams per year. Our present rate of warming is much greater than the rate of warming during the PETM (55.5 MYA). The best candidates for the source of CO2 release that caused the PETM are volcanoes, the oceans, and the permafrost. Methane hydrates (a solid form of methane found in low-temperature, high-pressure waters) might also be another factor.
The PETM and time after allowed for the optimal evolution of primates. That time might be called the Age of the Primates (analogous to the Age of the Dinosaurs). Figure 13 shows that the arrival of the Oligocene was accompanied by much lower temperatures resulting from CO2 drawdown. This decimated the habitat of primates except in the tropic rainforests. Primates disappeared completely from North America but thrived in Africa. Tectonic forces caused the generation of the African Rift Valley, characterized by diverse geology and a less homogenous and more fractured landscape which again provided new challenges but also new habitats for the evolution of primates and the eventual appearance of Homo Sapiens.
Update from December 3, 2023, Science
In December 2023, a refinement of CO2, temperature, and sea level rise data since the demise of the dinosaurs 65 million years ago was published. (The geological interval from 66 million years ago to now is called the Cenozoic Era and consists of 3 periods: the Paleogene until 23 MYA, the Neogene until 2.6 MYA, and the Quaternary.) A group, the Cenozoic Carbon Dioxide Proxy Integration Project (CenCO2PIP) Consortium, reviewed and reevaluated all "proxy" measurements used to determine past climate conditions (we will discuss proxies using isotope ratios in ice cores and sediments as proxies for actual CO2, temperature, and sea level changes in the next few sections.) Figure \(\PageIndex{15}\) below shows the revised data, representing our best. For this work, pay close attention to graphs A (CO2), B (relative temperature changes), and C (sea level changes). Note that CO2 levels in 2022 were last seen 14 million years ago!
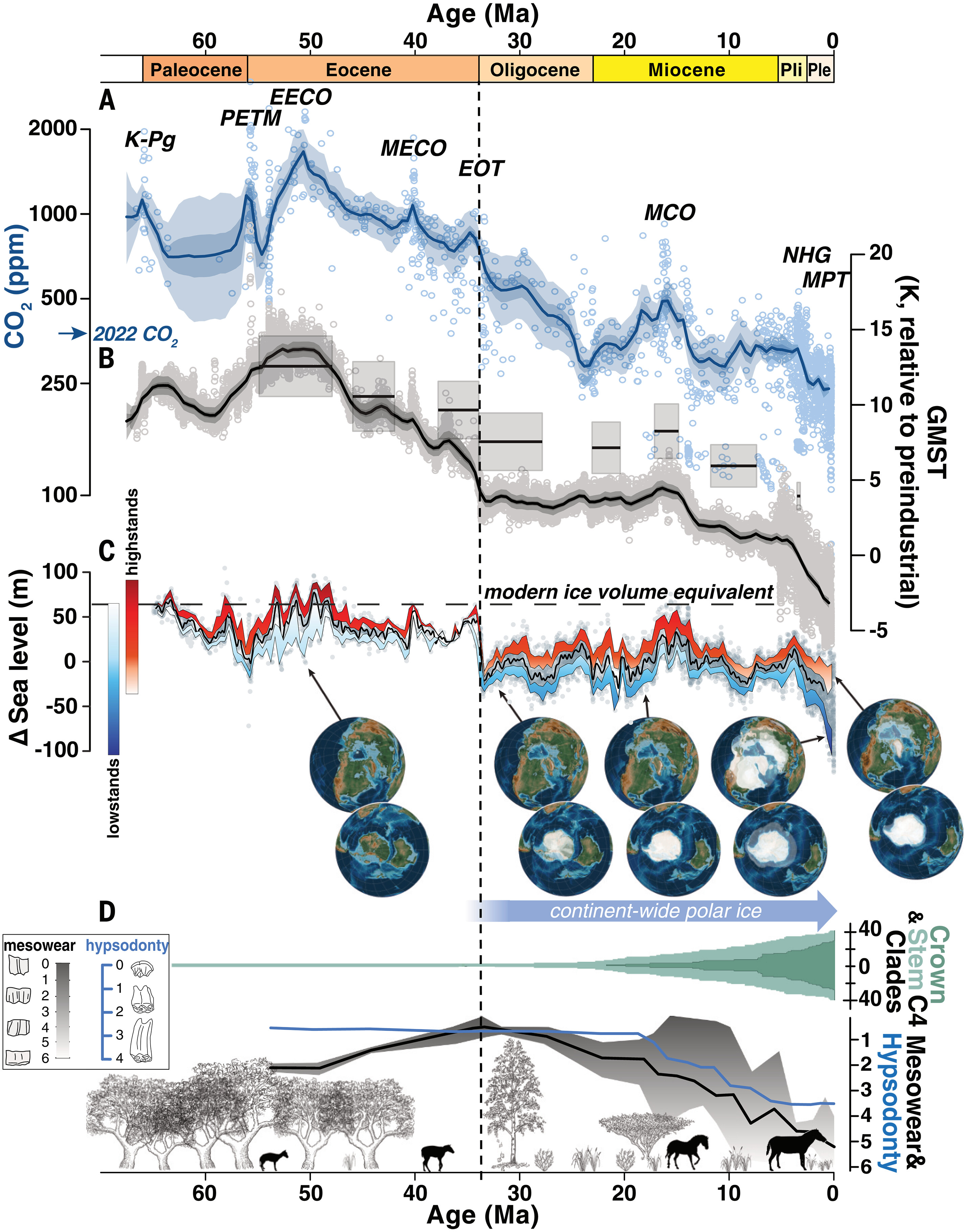
Figure \(\PageIndex{15}\): Category 1 paleo-CO2 record compared to global climate signals. From The Cenozoic CO2 Proxy Integration Project (CenCO2PIP) Consortium, Toward a Cenozoic history of atmospheric CO2.Science382, eadi5177(2023). DOI:10.1126/science.adi5177. Reprinted with permission from AAAS.
The legend below is directly taken from the Science article. The vertical dashed line indicates the onset of continent-wide glaciation in Antarctica.
(A) Atmospheric CO2 estimates (symbols) and 500-kyr mean statistical reconstructions (median and 50 and 95% credible intervals: dark and light-blue shading, respectively). Major climate events are highlighted: K-PG, Cretaceous/Paleogene boundary; PETM, Paleocene Eocene Thermal Maximum; EECO, Early Eocene Climatic Optimum; MECO, Middle Eocene Climatic Optimum; EOT, Eocene/Oligocene Transition; MCO, Miocene Climatic Optimum; NHG, onset of Northern Hemisphere Glaciation; and MPT, Mid-Pleistocene Transition. The 2022 annual average atmospheric CO2 of 419 ppm is indicated for reference.
(B) Global mean surface temperatures estimated from benthic δ18O data following Westerhold et al. (solid line, individual proxy estimates as symbols, and statistically reconstructed 500-kyr mean values shown as the continuous curve, with 50 and 95% credible intervals) and from surface temperature proxies (gray boxes).
(C) Sea level after with gray dots displaying raw data; the solid black line reflects median sea level in a 1-Myr running window. High- and lowstands are defined within a running 400-kyr window, with lower and upper bounds of highstands defined by the 75th and 95th percentiles and lower and upper bounds of lowstands defined by the 5th and 25th percentiles in each window. Globes depict select paleogeographic reconstructions and the growing presence of ice sheets in polar latitudes.
(D) Crown ages show that C4 clades, with CCMs adapted to low CO2, initially diversified in the early Miocene, and then rapidly radiated in the late Miocene. Flora transition from dominantly forested and woodland to open grassland habitats based on fossil phytolith abundance data. North American equids typify hoofed animal adaptations to new diet and environment, including increasing tooth mesowear (black line; note the inverted scale), hypsodonty (blue line), and body size.
The results reconfirm the strong correlation across geologic time of CO2 and temperature, but there are some time intervals when they were out of synchrony. One example is from 37 to 34 MYA, around the Eocene to Oligocene transition (EOT), described above. In Figure 15, CO2 didn't change much as the Earth cooled in the time preceding the EOT. In contrast, there was a big drop in CO2 in the Oligocene as the temperatures remained flat. The start of the Oligocene also showed a big drop (55 m) in sea levels coincident with the EOT, the fall in temperatures, and the rise in glaciation. The authors state that some of the divergences in CO2 and temperatures are likely not related directly to CO2, but other variables, such as changes in "paleogeography" arising from continental drift, plate tectonics, etc, that might have changed the ocean currents and also changes in reflectivity of sunlight. In addition, the proxies used for those times might have been affected by unaccounted-for changes in seawater composition and pH.
This extensive data presented an opportunity of calculate the climate sensitivity (how much temperature changes with a doubling of CO2) as we discussed above. The value calculated by Tierney was about 3.4 °C (6.1 °F), although Hansen's recent estimate is higher. This particular value is the equilibrium climate sensitivity (ECS), which includes relatively fast feedbacks like cloud cover, sea ice, etc, which act over 100s of years. The value calculated in the new study is not as fine (it's courser) with resolutions in 1000s of years, so instead, they calculate a much longer-term climate sensitivity called the Earth Systems Sensitivity (ESS) that includes long-term (in geological time) events such as changes in continent-wide ice sheets. The value of ESS is between 5°C (9°F) to 8°C (14.4°F), much higher than the ECS widely accepted for our present time. A doubling of CO2 without some mechanism for drawdown, if sustained over geological time, would transform the world of the distant future. It would be similar to conditions during the Paleocene/Eocene thermal maximum (PETM) when the poles warmed to near 70 0F, which allowed the presence of alligators and palm trees at the poles.
This chapter is divided into two sections. Read Chapter 01B, "Back to the Present and Future of Climate Change," to see where we are and where we are headed!
Summary
Chapter Summary
This chapter introduces the fundamental science behind climate change, focusing on how human activities have altered the Earth’s energy balance and disrupted long-established climate patterns. Key points include:
-
Historical Foundations and Urgency:
- Early studies by Eunice Foote and John Tyndall laid the groundwork for understanding the greenhouse effect.
- Modern climate models (developed since the 1980s) and recent observations confirm that anthropogenic CO₂ emissions are driving rapid global warming, necessitating immediate and aggressive action.
-
The Greenhouse Effect and Energy Imbalance:
- Earth’s climate is maintained by a balance between incoming solar radiation and outgoing infrared radiation.
- Greenhouse gases such as CO₂, methane (CH₄), and nitrous oxide (N₂O) absorb infrared radiation, effectively insulating the planet.
- Pre-industrial CO₂ levels (~280 ppm) have risen to 427 ppm as of January 2025, upsetting this balance and storing excess energy primarily in the oceans.
- The current energy imbalance is significant—equivalent to detonating several Hiroshima-type atomic bombs every second—illustrating the unsustainability of current trends.
-
Global Warming Potential (GWP):
- GWP is a metric used to compare the warming effects of different greenhouse gases in CO₂-equivalents over a 100-year period.
- Although CH₄ is more potent on a per-mass basis over short time frames, CO₂ remains the principal concern due to its long atmospheric lifetime and cumulative impact.
-
Climate Variability Over Geological Time:
- Ice core data reveal that while CO₂ and temperature have fluctuated naturally over the past 800,000 years, the recent rapid rise in CO₂ levels is unprecedented.
- The chapter discusses glacial-interglacial cycles, highlighting how relatively small changes in CO₂ (100 ppm) historically corresponded with major shifts in global climate.
-
Orbital Forcing and Feedback Mechanisms:
- The Milankovitch cycles (changes in Earth’s eccentricity, obliquity, and precession) initiate small temperature increases that trigger deglaciation.
- These temperature rises lead to the release of CO₂ from the oceans, amplifying warming through positive feedback loops.
- Dust feedbacks are also explored, showing how variations in dust deposition can either enhance or mitigate warming by influencing phytoplankton growth and planetary albedo.
-
Long-Term Climate Change and Carbon Cycle Interactions:
- The chapter reviews data spanning from 66 million years ago to the present, illustrating how CO₂ levels and global temperatures have paralleled each other over geological time.
- Major events such as the Paleocene–Eocene Thermal Maximum (PETM) are discussed, providing context for the rapid changes occurring today.
- The interactions between atmospheric CO₂ and ocean chemistry are detailed through chemical equilibria, explaining shifts in ocean pH and alkalinity that accompany changes in CO₂ concentrations.
-
Implications for Future Climate:
- Updated data indicate that equilibrium climate sensitivity (ECS) and the longer-term Earth Systems Sensitivity (ESS) may be higher than previously estimated, suggesting that a doubling of CO₂ could eventually raise global temperatures by 5–8 °C.
- The chapter concludes by emphasizing the critical need to address CO₂ emissions to avoid future climate scenarios that could jeopardize human habitability and ecosystem stability.
Overall, this chapter provides a comprehensive framework that combines historical context, physical principles, and biochemical processes to explain current climate change. It lays the groundwork for understanding how climate dynamics are interconnected with global biogeochemical cycles, setting the stage for further exploration of climate mitigation and adaptation strategies in subsequent sections.