14.1.6: Paired Tetrahedral Silicates
- Page ID
- 18651
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- lawsonite CaAl2Si2O7(OH)2•H2O
- epidote Ca2(Al,Fe)3Si3O12(OH)
- zoisite Ca2Al3Si3O12(OH)
- clinozoisite Ca2Al3Si3O12(OH)
- vesuvianite Ca10(Mg,Fe)2Al4Si9O34(OH)4
Mineralogists often group lawsonite, epidote, clinozoisite, and vesuvianite because they all contain pairs of SiO4 tetrahedra sharing a single bridging oxygen. Åkermanite and gehlenite, rare minerals belonging to the melilite group, are also paired tetrahedral silicates but are not considered in detail in this book.
In lawsonite all silica tetrahedra are paired. However, in epidote, clinozoisite, and vesuvianite, structures are more complicated because some SiO4 tetrahedra are unpaired. For this reason, these three minerals are sometimes not considered to be “true” paired tetrahedral silicates.
For more general information about paired tetrahedral silicates Section 6.4.
Lawsonite CaAl2Si2O7(OH)2•H2O
Origin of Name
Named after A. C. Lawson (1861–1952), a professor at the University of California.


Hand Specimen Identification
Occurrence in high-pressure metamorphic rocks, hardness, light color, and bladed or blocky character help identify lawsonite, but it is often very fine grained. The two photos seen here show coarse lawsonite crystals from the Franciscan terrane of California.
Physical Properties
| hardness | 8 |
| specific gravity | 2.1 |
| cleavage/fracture | perfect (100) and (010), fair {101}/uneven |
| luster/transparency | greasy, vitreous/transparent |
| color | typically gray, white, pale blue, or colorless |
| streak | white |
Properties in Thin Section
Lawsonite is usually colorless and exhibits high relief and interference colors no higher than first-order red. Biaxial (+), α = 1.665 , β = 1.674, γ = 1.685, δ = 0.020, 2V = 76° to 86°
Crystallography
Lawsonite is orthorhombic, a = 8.90, b = 5.76, c = 13.33, Z = 4; space group \(C\dfrac{2}{c}\dfrac{2}{m}\dfrac{2}{m}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Lawsonite may form tabular or prismatic crystals. Simple or lamellar twins are common.
Structure and Composition
Lawsonite is the only relatively common silicate in which all (SiO4) tetrahedra are paired, thus producing Si2O7 groups. The Si2O7 groups link Al(O,OH) octahedra; Ca occupies holes between the Si2O7 groups and the octahedra. Minor amounts of Ti, Fe, Mg, Na, and K may be present, but no major solid solutions are known.
Occurrence and Associations
Lawsonite is a metamorphic mineral typical of the blueschist facies. Associated high-pressure minerals include glaucophane, jadeite, pumpellyite, or aragonite. Other associated minerals include chlorite, plagioclase, titanite, quartz, and epidote.
Related Minerals
Hemimorphite, Zn4(Si2O7)(OH)2•H2O, and ilvaite, CaFe3O(Si2O7)(OH), are other paired tetrahedral silicates in which all SiO4 tetrahedra are paired. Other minerals usually considered in the same group are epidote, Ca2(Al,Fe)3Si3O12(OH); clinozoisite, Ca2Al3Si3O12(OH); piemontite, Ca2(Al,Mn)3Si3O12(OH); allanite, (Ca,Ce)2(Al,Fe,Mg)3Si3O12(OH); and vesuvianite, Ca10(Mg,Fe)2Al4Si9O34(OH,F)4.
Epidote Ca2(Al,Fe)3Si3O12(OH)
Origin of Name
From the Greek word epididonai, meaning “increase,” referring to the base of an epidote prism, one side of which is longer than the other.
Hand Specimen Identification
Epidote is most easily identified by its pistachio-green color (although some epidote is darker green), prismatic habit, and sometimes by its occurrence as an alteration product or accessory mineral. It is often very fine-grained or massive. The photo in Figure 14.214 below shows a rock made entirely of fine-grained epidote. The other photos show coarser specimens exhibiting well-developed prismatic character.




Physical Properties
| hardness | 6 to 7 |
| specific gravity | 3.4 to 3.5 |
| cleavage/fracture | perfect (001), poor (100)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | pistachio-green, less commonly yellow-green to dark green or black |
| streak | white |
Properties in Thin Section
In thin section, epidote has high relief and is usually colorless, but may be light green or pink and pleochroic, depending on composition. Interference colors range up to third order as Fe content increases. Football-shaped grains with concentric interference color rings are diagnostic of epidote. Epidote is biaxial (-), a = 1.71, to 1.75 , β = 1.72 to 1.78, γ = 1.73 to 1.80, δ = 0.01 to 0.05, 2V = 90° to 115°
Crystallography
Epidote is monoclinic, a = 8.98, b = 5.64, c = 10.22, β = 115.4°, Z = 2; space group \(P\dfrac{2_{1}}{m}\); point group \(\dfrac{2}{m}\).
Habit
Epidote crystals are usually prismatic elongated parallel to b, with faces showing striations. Fibrous or acicular crystals are common, too. Granular, massive, and fibrous aggregates are common.
Structure and Composition
Epidote is usually considered a paired tetrahedral silicate, but contains both paired and unpaired (SiO4) tetrahedra. Chains of edge-sharing Al(O,OH)6 octahedra, are linked by Si2O7 and SiO4 groups. Ca occupies large sites between the various groups. A complete solid solution exists between Fe-epidote, Ca2Fe3Si3O12(OH) and clinozoisite, Ca2Al3Si3O12(OH). Limited substitution exists between epidote and piemontite, Ca2(Mn,Al)3Si3O12(OH). Cr, Pb, V, Sr, Sn, and rare earths may be present in small amounts.
Occurrence and Associations
Epidote is a common and widespread mineral, characteristic of low- to medium-grade metabasites and marbles. Associated minerals include actinolite, chlorite, and albite in mafic rocks and diopside, grossular, and vesuvianite in marbles. Epidote is also produced by alteration of feldspar, pyroxene, and amphibole.
Varieties
Pistacite is a name sometimes used for pistachio green epidote. Allanite is an epidote mineral rich in rare earth elements. Hancockite is a pink Pb-rich variety of epidote. Sausserite is a name given to fine-grained epidote produced by alteration of plagioclase. Sr-rich epidote is called epidote-(Sr).
Related Minerals
Epidote is similar to other minerals of the epidote group. The group includes epidote, clinozoisite, allanite, piemontite, a rare-earth mineral, and about a dozen other minerals. All are monoclinic minerals that contain paired (SiO4) tetrahedra.
Zoisite Ca2Al3Si3O12(OH)
Origin of Name
Named after Baron von Zois (1747–1819), an Austrian who financed mineralogists.


Hand Specimen Identification
Occurrence in medium-grade metamorphic rocks is a key to identification. Zoisite often forms prismatic translucent or transparent crystals with vitreous or pearly lusters. Zoisite comes in a variety of colors and so can be confused with other minerals. The most common hues are white to gray, green, or green-brown. The crystals in these photos show examples from Baltistan, Pakistan.
Physical Properties
| hardness | 6 to 7 |
| specific gravity | 3.1 to 3.36 |
| cleavage/fracture | Perfect {010} imperfect {100}/uneven |
| luster/transparency | vitreous, pearly on cleavage surfaces/transparent to translucent |
| color | white, greenish gray, greenish brown, blue, purple, or pink |
| streak | white |
Properties in Thin Section
In thin section, zoisite has high relief, and is usually colorless and commonly shows dispersion. Interference colors are often anomalous; birefringence is low. Biaxial (+), α = 1.69, to 1.70 , β = 1.69 to 1.70, γ = 1.70 to 1.72, δ = 0.006 to 0.018, 2V = 0° to 70°.
Crystallography
Zoisite is orthorhombic, a = 8.94, b = 5.61, c = 10.23, Z = 2; space group \(P\dfrac{2}{n}\dfrac{2}{m}\dfrac{2}{a}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Zoisite generally forms prismatic crystals that display striations. Columnar crystals , sometimes with terminating pyramids, are typical but it may also be massive
Structure and Composition
The zoisite structure is similar to epidote’s, containing both paired and unpaired (SiO4) tetrahedra, but it is overall orthorhombic.
Occurrences and Associations
Zoisite occurs in schists and other medium-grade metamorphic rocks and in pegmatites, less commonly, in eclogites or blueschists.


Varieties
Tanzanite is blue to purple gem quality zoisite (Figure 14.220). Thulite is a pink variety (Figure 14.221).
Related Minerals
Clinozoisite has a closely related structure but is monoclinic. Zoisite is structurally and chemically related to all the other paired tetrahedral silicates (see lawsonite related minerals).
Clinozoisite Ca2Al3Si3O12(OH)
Origin of Name
Named after zoisite, its orthorhombic polymorph.


Hand Specimen Identification
Clinozoisite is difficult to identify in hand specimen. It is most commonly green, like the specimens in these two photos, but various other colors are possible. When green, it may be mistaken for epidote; if uncolored or lightly colored, it is often overlooked. It cannot be distinguished with certainty from zoisite (orthorhombic) without X-ray analysis.
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 3.1 to 3.4 |
| cleavage/fracture | perfect (001)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | light to dark green, yellow, or gray |
| streak | white |
Properties in Thin Section
Clinozoisite has high relief, and is usually colorless. Interference colors are often anomalous; birefringence is low. Biaxial (+), α = 1.67, to 1.72 , β = 1.67 to 1.72, γ = 1.69 to 1.73, δ = 0.005 to 0.015, 2V = 14° to 90°
Crystallography
Clinozoisite is monoclinic, a = 8.94, b = 5.61, c = 10.23, β = 115.0°, Z = 2; space group \(P\dfrac{2_{1}}{m}\); point group \(\dfrac{2}{m}\).
Habit
Crystals of clinozoisite are prismatic, fibrous, or acicular, usually elongated parallel to the b-axis, with faces showing striations. Granular, massive, and fibrous aggregates are common.
Structure and Composition
The clinozoisite structure is similar to epidote’s, containing both paired and unpaired (SiO4) tetrahedra (see epidote). A complete solid solution exists between Fe-epidote, Ca2Fe3Si3O12(OH) and clinozoisite, Ca2Al3Si3O12(OH).
Occurrence and Associations
Clinozoisite, like epidote, is a product of metamorphism of Ca-rich rocks. It forms instead of epidote in relatively Fe-poor rocks.

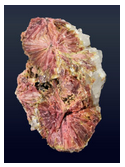

Varieties
Clinozoisite comes in many different colors besides green. Several examples are seen in these three photos. Pink varieties are called clinothulite.
Related Minerals
A polymorph of clinozoisite, named simply zoisite, is orthorhombic. Clinozoisite is structurally and chemically related to all the other paired tetrahedral silicates (see lawsonite related minerals).
Vesuvianite (Idocrase) Ca10(Mg,Fe)2Al4Si9O34(OH)4
Origin of Name
From the Italian Mount Vesuvius locality where this mineral was found.


Hand Specimen Identification
Occurrence in contact metamorphic rocks, translucent character, tetragonal or columnar habit, and brown or greenish color help identify vesuvianite. If not euhedral, identification can be problematic. It is sometimes confused with epidote, tourmaline, or grossular garnet. Figures 14.227 and 14.228 show examples.
Physical Properties
| hardness | 6.5 |
| specific gravity | 3.4 |
| cleavage/fracture | poor (001), (100), {110}/subconchoidal |
| luster/transparency | vitreous, resinous/transparent |
| color | brown, also yellow, green, blue, pink, or red |
| streak | white |
Properties in Thin Section
Vesuvianite is usually colorless but may be pleochroic in light green, brown, or yellow. High relief, low birefringence, and anomalous interference colors are typical. It may be confused with zoisite and clinozoisite but is uniaxial and lacks a good prismatic cleavage. Uniaxial (-), ω = 1.706, ε = 1.701, δ = 0.005.
Crystallography
Vesuvianite is tetragonal, a = 15.66, c = 11.85, Z = 4; space group \(P\dfrac{4}{n}\dfrac{2}{n}\dfrac{2}{c}\); point group \(\dfrac{4}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Typical vesuvianite occurs as coarse, prismatic, brown tetragonal prisms. Faces may be striated and crystals may be terminated by pyramids. Crystals may combine to form striated columnar masses, fibrous sheaves, or granular aggregates.
Structure and Composition
Vesuvianite, usually considered a paired tetrahedral silicate, contains both paired and unpaired (SiO4) tetrahedra. Its structural similarity to grossular leads to some misidentification. Composition is highly variable. Mn, Na, and K may replace Ca. Ti and Al may replace (Mg,Fe). Other elements, including B, Be, Cr, Cu, Li, Zn, and rare earths, may be present.
Occurrence and Associations
Vesuvianite is found primarily in contact aureoles associated with impure limestones or dolomites. Associated minerals include garnet, wollastonite, epidote, diopside, and carbonates. It is also found in altered mafic rocks, including serpentinites.

Varieties
Cyprine is a blue variety of vesuvianite (Figure 14.229)
Related Minerals
Vesuvianite is structurally and chemically similar to the garnet grossular; it is less similar to epidote and other paired tetrahedral silicates.


