14.1.5: Silicate Class - Isolated Tetrahedral Silicates
- Page ID
- 18649
14.1.5.1 Garnets
Garnet Group Minerals
Pyralspite Series
pyrope Mg3Al2Si3O12
almandine Fe3Al2Si3O12
spessartine Mn3Al2Si3O12
Ugrandite Series
grossular Ca3Al2Si3O12
andradite Ca3Fe2Si3O12
uvarovite Ca3Cr2Si3O12
Garnet composition is quite variable but all garnets have chemical formula A3B2Si3O12. Their structures consist of isolated (SiO4)4- tetrahedra linked to distorted octahedrons (B atoms; most commonly Al3+ or Fe3+) and to distorted dodecahedrons (A atoms; most commonly Ca2+, Mg2+, Fe2+, or Mn2+). Mineralogists conveniently divide garnets into two series, the pyralspites (pyrope–almandine-spessartine) and the ugrandites (uvarovite-grossular–andradite). Complete solid solution exists within each series, but only limited solid solution occurs between the two.
Garnets are nearly exclusively found in metamorphic rocks. Chapter 8 discusses many different aspects of garnet occurrences.
Pyrope Mg3Al2Si3O12
Origin of Name
From the Greek word pyropos, meaning “fiery,” a reference to this mineral’s luster.


Hand Specimen Identification
Hardness, lack of cleavage, vitreous luster, and crystal shape (when euhedral) identify garnets. Some varieties have distinct colors, and different species can sometimes be identified by color or association. Pyrope (Mg-garnet) is most often red. It can be mistaken for almandine, the most common kind of red garnet. Garnets are complex solid-solution minerals, and determining exact composition requires analytical data.
Physical Properties
| hardness | 7 |
| specific gravity | 3.54 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/often translucent to transparent |
| color | red and occasionally other colors including black |
| streak | white |
Properties in Thin Section
Pyrope is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. It is isotropic, n = 1.71.
Crystallography
Pyrope is cubic, a = 11.46, Z = 8; space group \(I\dfrac{4}{m}\overline{3}\dfrac{2}{m}\); point group \(\dfrac{4}{m}\overline{3}\dfrac{2}{m}\).
Habit
Equant grains, sometimes displaying dodecahedral or trapezohedral faces, characterize pyrope and other garnets. Massive occurrences are rare.
Structure and Composition
The name pyrope refers to garnets close in composition to the Mg end member of the pyralspite series. The structure of pyrope is similar to the structures of other garnets.
Occurrence and Associations
Pyrope is only stable in high-pressure rocks and is found in eclogites and other mafic and ultramafic rocks from deep within Earth. Commonly associated minerals include olivine, pyroxene, spinel, and occasionally diamond.
Almandine Fe3Al2Si3O12
Origin of Name
From Alabanda, a Middle Eastern trade center where garnets were cut and polished in the first century A.D.



Hand Specimen Identification
Hardness, lack of cleavage, luster, and crystal shape (when euhedral) identify garnets. Garnets are, however, solid-solution minerals, and determining exact composition requires analytical data. Some varieties have distinct colors, and different species can sometimes be distinguished by color or association. Almandine typically has a distinctive deep wine-red color that can be seen in the three photos above. In Figure 14.156, the almandine is accompanied by green diopside and dark purplish clinochlore. This pretty specimen comes from the Aosta Valley, Italy.
Physical Properties
| hardness | 7 |
| specific gravity | 4.33 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/often translucent to transparent |
| color | deep red |
| streak | white |
Properties in Thin Section
Almandine is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. It is isotropic, n = 1.83.
Crystallography
Almandine, like all garnets, is cubic, a = 11.46, Z = 8; space group \(I\dfrac{4}{m}\overline{3}\dfrac{2}{m}\); point group \(\dfrac{4}{m}\overline{3}\dfrac{2}{m}\).
Habit
Almandine is characterized by euhedral to subhedral equant grains displaying dodecahedral or trapezohedral faces. Massive occurrences are rare.
Composition and Structure
Almandine, the Fe end member of the garnet pyralspite series, may contain appreciable amounts of Ca, Mg, or Mn replacing Fe. It has the same structure as other garnets.
Occurrence and Associations
Almandine is a common mineral in medium- and high-grade metamorphic rocks. It is often found with quartz, feldspars, micas, staurolite, cordierite, chloritoid, tourmaline, and kyanite or sillimanite.
Spessartine Mn3Al2Si3O12
Origin of Name
From Spessart, a district in Germany.

Hand Specimen Identification
Hardness, lack of cleavage, luster, and crystal shape (when euhedral) identify garnets. Some varieties have distinct colors, but spessartine does not. It usually can only be identified by chemical analysis, although association with other Mn minerals is suggestive. Figure 14.157 shows a mass of euhedral spessartine crystals from Fujian Province, China.
Physical Properties
| hardness | 7 |
| specific gravity | 4.19 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/ commonly translucent to transparent |
| color | red, reddish orange, yellowish brown, reddish brown, or brown |
| streak | white |
Properties in Thin Section
Spessartine is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. Isotropic, n = 1.80.
Crystallography
Spessartine, like all garnets, is cubic, a = 11.62, Z = 8; space group \(I\dfrac{4}{m}\overline{3}\dfrac{2}{m}\); point group \(\dfrac{4}{m}\overline{3}\dfrac{2}{m}\).
Habit
Euhedral to subhedral equant grains displaying dodecahedral or trapezohedral faces characterize spessartine. Massive occurrences are rare.
Structure and Composition
Spessartine is the Mn end member of the pyralspite series. Natural spessartine may contain appreciable amounts of Ca, Mg, or Fe replacing Mn. Spessartine has the same structure as other garnets.
Occurrence and Associations
Spessartine is found with other Mn minerals in Mn-rich skarns, low-grade metamorphic rocks, some rare granites and rhyolites, and, occasionally, in pegmatites. Common associated minerals in igneous rocks are quartz, feldspars, and micas.
Grossular Ca3Al2Si3O12
Origin of Name
From grossularia, the Latin name for the pale green gooseberry, which is the same color as some grossular.



Hand Specimen Identification
Like all garnets, grossular is hard, commonly vitreous, forms equant crystals and has no cleavage. Grossular is typically rose-colored, pink, red-pink, or olive-green. Its usual occurrence in metacarbonate rocks helps identification. If an unusual color, compositional data may be needed to distinguish it from other garnets.
The grossulars shown in Figures 14.158 and 14.159 display the most common hues for grossular. The emerald-green tsavorite in Figure 14.160 is an unusual but spectacular variety of grossular.
Physical Properties
| hardness | 6.5 |
| specific gravity | 3.56 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/translucent to transparent |
| color | rose, pink, and olive green colors are most common; also brown or yellow |
| streak | white |
Properties in Thin Section
Grossular is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. Isotropic, n = 1.75.
Crystallography
Grossular, like all garnets, is cubic, a = 11.85, Z = 8; space group \(I\dfrac{4}{m}\overline{3}\dfrac{2}{m}\); point group \(\dfrac{4}{m}\overline{3}\dfrac{2}{m}\).
Habit
Grossular is characterized by euhedral to subhedral equant grains displaying dodecahedral or trapezohedral faces. Massive occurrences are rare.
Structure and Composition
Grossular is the Al end member of the ugrandite series, Ca3(Fe3+,Al3+,Cr3+)2Si3O12, but natural grossular may contain appreciable amounts of Fe3+ or Cr3+ in solid solution. The structure is the same as other garnets.
Occurrence and Associations
Grossular is found in marbles where it may be associated with calcite, dolomite, quartz, tremolite, diopside, and wollastonite.
Varieties
Hydrogrossular, or hibschite, is the name for grossular in which a substantial amount of Si4+ has been replaced by 4H+. Tsavorite (the green variety seen above in Figure 14.160) gets it green color from trace amounts of vanadium or chromium.
Andradite Ca3Fe2Si3O12
Origin of Name
Named after J. B. d’Andrada e Silva (1763–1838), a Portuguese mineralogist.


Hand Specimen Identification
Crystal shape, lack of cleavage, luster, and color identify garnets. The different species can sometimes be inferred by color or association. Andradite‘s color is quite variable but is generally yellow, green or brown. Identifying it with certainty requires analytical data.
Figure 14.161 shows a green gemmy variety of andradite called demantoid. It gets its deep color from trace amounts of Cr in its structure. A small amount of stilbite is also present in the photo. Figure 14.162 shows more typical andradite crystals from Mali.
Physical Properties
| hardness | 7 |
| specific gravity | 3.86 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous, sometimes pearly/translucent to transparent |
| color | yellow, brown, green |
| streak | white |
Properties in Thin Section
Andradite is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. Isotropic, n = 1.87.
Crystallography
Andradite, like all garnets, is cubic, a = 12.05, Z = 8; space group \(I\dfrac{4}{m}\overline{3}\dfrac{2}{m}\); point group \(\dfrac{4}{m}\overline{3}\dfrac{2}{m}\).
Habit
Andradite is characterized by euhedral to subhedral equant grains displaying dodecahedral or trapezohedral faces. Massive occurrences are rare.
Structure and Composition
Andradite, the Fe3+ end member of the garnet ugrandite series, may contain appreciable amounts of Al3+ or Cr3+ replacing Fe3+. It has the same structure as other garnets.
Occurrence and Associations
Andradite is found in marbles and occasionally as an accessory mineral in igneous rocks. Typical associated minerals include hedenbergite and magnetite.
Varieties
Melanite is a black variety of andradite. Demantoid (Figure 14.161) is a gemmy green variety of andradite.
Uvarovite Ca3Cr2Si3O12
Origin of Name
Named after Count S. S. Uvarov (1785–1855), president of the St. Petersburg Academy in Russia.

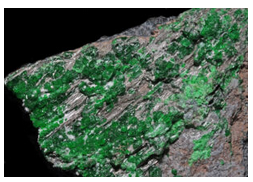
Hand Specimen Identification
Crystal shape, lack of cleavage, luster, and hardness identify garnets. The different species can sometimes be inferred by color or association. Uvarovite, like some other chrome minerals, often has a strong emerald green color. But, so do varieties of some other garnet species. Uvarovite‘s occurrence in ultramafic rocks provides hints to its Cr-rich composition, but identifying uvarovite with certainty requires compositional information.
Figure 14.163, above, shows several large uvarovite crystals in a rock from western Finland. Figure 14.164 shows small crystals of the same mineral from the Saranovskii Mine, a classic collecting spot in the central Ural Mountains, Russia.
Physical Properties
| hardness | 7.5 |
| specific gravity | 3.80 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/translucent |
| color | emerald green |
| streak | white |
Properties in Thin Section
Uvarovite is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. Isotropic, n = 1.85.
Crystallography
Uvarovite, like all garnets, is cubic, a = 12.00, Z = 8;
Habit
Uvarovite is characterized by euhedral to subhedral equant grains displaying dodecahedral or trapezohedral faces. Massive occurrences are rare.
Structure and Composition
Uvarovite, the Cr3+ end member of the garnet ugrandite series, may contain appreciable amounts of Fe3+, or Al3+ replacing Cr3+. It has the same structure as other garnets.
Occurrence and Associations
Uvarovite is a rare mineral found primarily in peridotites and often associated with chrome ore. It is more rarely found in metamorphic rocks. In peridotites, it is typically found with chromite, olivine, pyroxene, and serpentine.
14.1.5.2 Olivine Group Minerals
Olivine Group Minerals
forsterite Mg2SiO4
fayalite Fe2SiO4
tephroite Mn2SiO4
monticellite CaMgSiO4
Olivine, an abundant mineral in mafic and ultramafic igneous rocks, has the general formula (Mg,Fe,Mn)SiO4. Its structure is similar to that of garnet: isolated SiO4 tetrahedra are linked by divalent cations in octahedral coordination. Complete solid solution exists between the important end members forsterite (Mg-olivine) and fayalite (Fe-olivine), and, less important, tephroite (Mn-olivine). Limited solid solution toward a Ca2SiO4 end member is also possible, but the rare mineral larnite, with composition Ca2SiO4, does not have the olivine structure. Monticellite, CaMgSiO4, is grouped with the olivines, but because of its highly distorted structure is not considered a true olivine.
For more general information about the olivine group, see the olivine section in Chapter 5.
Forsterite Mg2SiO4
Origin of Name
Named after Jacob Forster, a scientist and founder of Heuland Cabinet.



Hand Specimen Identification
Olivine is distinguished by its glassy luster, conchoidal fracture, and usually olive-green color. Association and alteration to serpentine help identification sometimes. Olivines are occasionally confused with epidote or green pyroxene.
Forsterite is the name of end-member Mg2SiO4–olivine and also a general name used for any Mg-rich olivine. Distinguishing forsterite from other olivines is usually based on color (forsterite is often green but may be white if it contains little Fe) but certain identification requires optical or X-ray data.
Figure 14.165 shows the most common occurrence of visible forsterite crystals – as phenocrysts in a volcanic rock such as basalt. Figure 14.166 shows millimeter-sized forsterite phenocrysts that were removed from a basalt. Figure 14.167 is a photo of euhedral forsterite crystal that came from olivinite dikes on St. Johns Island in the Red Sea, Egypt.
Physical Properties
| hardness | 6.5 |
| specific gravity | 3.2 |
| cleavage/fracture | poor (010) and (100)/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | varies, white or green, less commonly yellow |
| streak | white |
Properties in Thin Section
Mg-rich olivines are colorless in thin section. Index of refraction and birefringence are high. Poor cleavage, irregular fracture, often equant grains, relatively high birefringence, and alteration to serpentine or chlorite help identification. Biaxial (+), α = 1.635 , β = 1.651, γ = 1.670, δ = 0.035, 2V = 85° to 90°.
Crystallography
Forsterite and other olivine minerals are orthorhombic, a = 4.78, b = 10.28, c = 6.00, Z = 4; space group \(P\dfrac{2_{1}}{b}\dfrac{2_{1}}{n}\dfrac{2_{1}}{m}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Rare euhedral olivine crystals are combinations of prisms and dipyramids, often having a tabular or lozenge shape. Granular forms that resemble green sand, or embedded grains, are common.
Structure and Composition
In olivine, isolated SiO4 tetrahedra are linked by MgO6 octahedra. Complete solid solution exists between forsterite (Mg2SiO4), fayalite (Fe2SiO4), and tephroite (Mn2SiO4). Minor Ca or Ni may also be present as replacement for Mg.



Occurrence and Associations
Forsterite, a primary mineral in many mafic and ultramafic rocks, is typically associated with pyroxenes, plagioclase, spinel, garnet, and serpentine. Figures 14.168 and 14.169 show two examples of forsterite in dunites, olivine-dominated ultramafic rocks.
Less commonly, forsterite is found as a metamorphic mineral. For example, Figure 14.170 shows green forsterite crystals in a marble. Even less commonly, forsterite is found in young sediments

Varieties
Peridot (Figure 14.171) is a gemmy green transparent variety of forsterite.
Related Minerals
The principal olivine end members are forsterite, fayalite, and tephroite. Olivine is isostructural with chrysoberyl, BeAl2O4. Minerals with similar but not identical structures include monticellite (CaMgSiO4), sinhalite (MgAlBO4), larnite (Ca2SiO4), and kirschsteinite (CaFeSiO4).
Fayalite Fe2SiO4
Origin of Name
Named after Fayal Island of the Azores, where fayalite was once found.


Hand Specimen Identification
Common olivine is distinguished by its glassy luster, conchoidal fracture, and usually olive-green color. In the absence of compositional data, we assume any green olivine to be Mg-rich, and thus call it forsterite. Fayalite (Fe-rich olivine), in contrast, is often in various shades of brown or yellow. Certain identification, however requires X-ray or optical data.
Figure 14.172 shows a small single crystal of brown fayalite, and Figure 14.173 shows a mass of fayalite crystals that range from brown to green in color.
Physical Properties
| hardness | 6.5 |
| specific gravity | 4.4 |
| cleavage/fracture | poor (010) and (100)/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | brown, yellow, or greenish-yellow |
| streak | white or yellow |
Properties in Thin Section
Fe-rich olivines are pale yellow or green in thin section and may be weakly pleochroic. Index of refraction and birefringence are high. Poor cleavage, often equant grains, and alteration to serpentine or chlorite help identification. Biaxial (-), α = 1.827 , β = 1.877, γ = 1.880, δ = 0.053, 2V = 47° to 54°.
Crystallography
Fayalite is orthorhombic, a = 4.81, b = 10.61, c = 6.11, Z = 4; space group \(P\dfrac{2_{1}}{b}\dfrac{2_{1}}{n}\dfrac{2_{1}}{m}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Rare euhedral fayalite crystals display combinations of prisms and dipyramids, often having a tabular or lozenge shape. Subhedral or anhedral embedded grains are common.
Structure and Composition
Fayalite structure is the same as that of other olivines: isolated SiO4 tetrahedra are linked by FeO6 octahedra. Complete solid solution exists between fayalite (Fe2SiO4), forsterite (Mg2SiO4), and tephroite (Mn2SiO4). Minor Ca or Ni may also be present as replacement for Fe.
Occurrence and Associations
Fayalite, less common than Mg-rich olivine, is found in some Fe-rich granitic igneous or metamorphic rocks.
Related Minerals
The principal olivine end members are forsterite, fayalite, and tephroite. Many other minerals have identical or related structures (see forsterite).
Monticellite CaMgSiO4
Origin of Name
Named after Italian mineralogist Teodoro Monticelli (1759–1846).



Hand Specimen Identification
Occurrence in high-temperature metacarbonates, association with other metacarbonate minerals and calcite, common brown color, conchoidal fracture, and habit help identify monticellite. It is difficult to distinguish from other olivine minerals without optical or X-ray data.
Figures 14.174 and 14.175 show photos of monticellite from the classic collecting site in Crestmore, California. In both, blue calcite accompanies the monticellite. Figure 14.176 shows a single crystal of transparent very light tan monticellite from a quarry near Koblenz, Germany. The monticellite crystal is on white calcite.
Physical Properties
| hardness | 5.5 |
| specific gravity | 3.15 |
| cleavage/fracture | poor (010) and (100)/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white, gray, or green |
| streak | white |
Properties in Thin Section
Monticellite is similar to olivine but has greater 2V. Biaxial (-), α = 1.645 , β = 1.655, γ = 1.665, δ = 0.020, 2V = 72° to 82°.
Crystallography
Monticellite is orthorhombic, a = 4.82, b = 11.08, c = 6.38, Z = 4; space group \(P\dfrac{2_{1}}{b}\dfrac{2_{1}}{n}\dfrac{2_{1}}{m}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Crystals tend to be subequant combinations of prisms and dipyramids. Monticellite is usually embedded grains or massive patches in a carbonate-rich host.
Structure and Composition
The structure of monticellite is similar to that of olivine, but the mismatch in size between Ca and Mg leads to some slight differences (see forsterite structure). Fe may substitute for Mg, leading to solid solutions with kirschsteinite, CaFeSiO4. Minor Al and Mn may also be present.
Occurrence and Associations
A rare mineral, monticellite is found in skarns and, less commonly, in regionally metamorphosed rocks. Associated minerals include calcite, forsterite, åkermanite, merwinite, and tremolite. Very minor occurrences have been reported from ultramafic igneous rocks.
Related Minerals
The true olivines (forsterite, fayalite, tephroite) are closely related to monticellite. Kirschsteinite, CaFeSiO4, is isostructural with monticellite. Minerals with similar but not identical structures include sinhalite, MgAl(BO4), and larnite, Ca2SiO4.
14.1.5.3 Humite Group Minerals
Humite Group Minerals
norbergite Mg3SiO4(OH,F)2
chondrodite Mg5(SiO4)2(OH,F)2
humite Mg9(SiO4)3(OH,F)2
clinohumite Mg9(SiO4)4(OH,F)2
The Humite Group contains about 10 minerals; the four Mg-humites listed in the blue box are the most important. Other minerals of the group contain Mn, Ca, or Zn in place of some or all the Mg.
All minerals of the humite group are isolated tetrahedral silicates with structural similarity to olivine. Their general formula is nMg2SiO4•Mg(OH,F)2, where n is 1, 2, 3, and 4, respectively, for norbergite, chondrodite, humite, and clinohumite. Chondrodite is the most common of the humite minerals. Humite is relatively rare; only the other three are described below. These minerals have similar compositions and share many properties, making it difficult to distinguish one from another.
Norbergite Mg3SiO4(OH,F)2
Origin of Name
Named after the type locality at Norberg, Sweden.


Hand Specimen Identification
The most common members of the humite group (norbergite, chondrodite, and clinohumite) cannot be distinguished without optical or X-ray data. They are usually identified by association, light color, and their form. They may be difficult to distinguish from olivine. The photos shown in Figures 14.177 and 14.198 show typical brown norbergite. In Figure 14.178, calcite accompanies the norbergite.
Physical Properties
| hardness | 6.5 |
| specific gravity | 3.16 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white, yellow, brown, red |
| streak | white |
Properties in Thin Section
Humite group minerals are biaxial (+). They may resemble olivine in thin section, but most olivines are biaxial (-). Additionally, humite-group minerals have lower birefringence than olivine. Norbergite is biaxial (+), α = 1.561 , β = 1.570, γ = 1.587, δ = 0.026, 2V = 44° to 50°.
Crystallography
Norbergite is orthorhombic, a = 4.70, b = 10.22, c = 8.72, Z = 4; space group \(P\dfrac{2_{1}}{b}\dfrac{2_{1}}{n}\dfrac{2_{1}}{m}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Norbergite crystals, usually found as isolated grains, are variable and display many forms. Highly modified orthorhombic or pseudoorthorhombic shapes are common.
Structure and Composition
Norbergite‘s structure consists of alternating layers of forsterite and brucite composition. Norbergite is usually close to end-member composition, although F:OH ratios are variable. Some Fe may replace Mg.
Occurrence and Associations
Norbergite is a rare mineral found in metamorphosed carbonate rocks. Associated minerals include calcite, dolomite, phlogopite, diopside, spinel, wollastonite, grossular, forsterite, and monticellite.
Chondrodite Mg5(SiO4)2(OH,F)2
Origin of Name
From the Greek word chondros meaning “grain,” referring to this mineral’s occurrence as isolated grains.



Hand Specimen Identification
The members of the humite group are usually identified by association, light color, and form. Chondrodite is difficult to distinguish from other humite group minerals and from olivine, even when viewed in thin section with an optical microscope.
Figure 14.179 shows a single crystal of euhedral chondrodite. But, this mineral more commonly occurs as disseminated subhedral to anhedral grains in marbles; Figures 14.180 and 14.181 are two examples of chondrodite-bearing marbles.
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 3.16 to 3.26 |
| cleavage/fracture | poor (100)/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white to yellow |
| streak | white |
Properties in Thin Section
Chondrodite and other humite group minerals resemble olivines in thin section, but most olivines are biaxial (-) instead of (+), and humites have lower birefringence. Biaxial (+), α = 1.60, β = 1.62, γ = 1.63, δ = 0.03, 2V = 60° to 90°.
Crystallography
Chondodrite is monoclinic, a = 4.73, b = 10.27, c = 7.87, β = 109.1°, Z = 2; space group \(P\dfrac{2_{1}}{b}\); point group \(\dfrac{2}{m}\).
Habit
Usually found as isolated grains, chondrodite crystals are variable and display many forms. Highly modified orthorhombic or pseudoorthorhombic crystals, with or without {001} twinning, are common.
Structure and Composition
Chondodrite‘s structure consists of layers of forsterite and brucite composition. Chondrodite contains two forsterite layers for each brucite layer. F:OH ratios are variable. Some Fe may replace Mg, and Ti content can be substantial.
Occurrence and Associations
Like norbergite, chondrodite is a rare mineral found in metamorphosed carbonate rocks. Associated minerals include calcite, dolomite, phlogopite, diopside, spinel, wollastonite, grossular, forsterite, and monticellite. Chondrodite has also been found in a few rare carbonatites.
Clinohumite Mg9(SiO4)4(OH,F)2
Origin of Name
Named after English mineralogist Sir Abraham Hume (1749–1839).


Hand Specimen Identification
The members of the humite group (norbergite, chondrodite, and clinohumite) cannot be distinguished from each other, and sometimes from olivine, without optical or X-ray data. They are usually identified by association, light color, and form. The photos show two views of clinohumite from Tajikistan. The stones in the photo on the left are advertised as gem quality rough; a parcel of 100 pieces was selling for $700 in 2022.
Physical Properties
| hardness | 6 |
| specific gravity | 3.21 to 3.35 |
| cleavage/fracture | poor (100)/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white to yellow |
| streak | white |
Properties in Thin Section
Clinohumite and other humite group minerals resemble olivines in thin section, but most olivines are biaxial (-), and humites have lower birefringence. Clinohumite is biaxial (+), α = 1.63 , β = 1.64, γ = 1.59, δ = 0.03 to 0.04, 2V = 73° to 76°.
Crystallography
Clinohumite is monoclinic, a = 4.75, b = 10.27, c = 13.68, β = 100.8°, Z = 2; space group \(P\dfrac{2_{1}}{b}\); point group \(\dfrac{2}{m}\).
Habit
Usually found as isolated grains, clinohumite crystals are variable and display many forms. Highly modified pseudoorthorhombic crystals with or without {001} twinning are common.
Structure and Composition
Clinohumite‘s structure is similar to the other humite group minerals (see chondrodite structure) except that the ratio of forsterite: brucite layers is 4:1. Some Fe may replace Mg, and F:OH ratios are variable. Ti is almost always present in small amounts.
Occurrence and Associations
The most significant occurrences of clinohumite are in metamorphosed carbonate rocks, similar to other humite-group minerals.

Varieties
Titanoclinohumite, an especially Ti-rich variety, has been reported from a few rare serpentinites and gabbros. Figure 14.184 shows red titanoclinohumite in a rock that also contains light-green clinochlore.
14.1.5.4 Aluminosilicates
Aluminosilicate Group Minerals
kyanite Al2SiO5
andalusite Al2SiO5
sillimanite Al2SiO5
The aluminosilicate polymorphs vary little from stoichiometric Al2SiO5 composition. All are isolated tetrahedral silicates but have distinctly different structures. In kyanite all the Al is in 6-fold coordination, in andalusite half is in 5-fold coordination and half is in 6-fold coordination, and in sillimanite half is in 4-fold coordination and half is in 6-fold coordination. The aluminosilicates are important minerals in pelitic metamorphic rocks. As discussed in Chapter 8, the presence of a particular polymorph indicates a general range of pressure-temperature at which the rock must have formed. For this reason, and because they are relatively common, the aluminosilicates are key metamorphic index minerals.
Kyanite Al2SiO5
Origin of Name
From the Greek word kyanos, meaning “blue.”

Hand Specimen Identification
Kyanite is brittle, forms splintery/bladed crystals, and is easily cleaved into acicular fragments. It is almost always some shade of blue, and there are few other blue minerals that form long bladed crystals. A blue color and occurrence in metamorphosed pelites or quartzites are adequate to identify it in most cases. Figure 14.185 shows blades of kyanite in a metaquartzite. Figure 3.64 shows other examples of splintery kyanite blades.
Physical Properties
| hardness | 5 to 7 |
| specific gravity | 3.60 |
| cleavage/fracture | two prominent: perfect (100), good (010)/uneven |
| luster/transparency | vitreous, sometime pearly/transparent to translucent |
| color | typically light to dark blue, may be white |
| streak | white |
Properties in Thin Section
Kyanite is typically colorless in thin section, but may be weakly blue and pleochroic. High relief, low birefringence, and excellent cleavage aid identification. It may be confused with sillimanite or andalusite, but sillimanite has a small 2V, and andalusite has parallel extinction. Kyanite is biaxial (-), α = 1.712 , β = 1.720, γ = 1.728, δ = 0.016, 2V = 82° to 83°.
Crystallography
Kyanite is triclinic, a = 7.10, b = 7.74, c = 5.57, α = 90.08°, β = 101.03°, γ = 105.73°, Z = 4; space group \(P\overline{1}\); point group \(\overline{1}\).
Habit
Kyanite is usually in long blade-shaped or tabular crystals, sometimes forming parallel or radiating aggregates.
Structure and Composition
In kyanite, chains of AlO6 octahedra are linked by additional AlO6 octahedra and by SiO4 tetrahedra. Kyanite is always near to Al2SiO5 composition; it may contain very minor Fe, Mn, or Cr.

Occurrence and Associations
Kyanite is primarily a metamorphic mineral found in medium- and high-pressure schists and gneisses. Figure 14.186 is a photo of a typical kyanite schist. Common associated minerals are quartz, feldspar, mica, garnet, corundum, and staurolite. Kyanite is also (rarely) found in aluminous eclogites and other rocks of deep origin.
Related Minerals
Kyanite has two polymorphs, andalusite and sillimanite.
Andalusite Al2SiO5
Origin of Name
Named for Andalusia, a province of Spain.

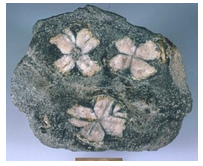

Hand Specimen Identification
Andalusite is found in metapelites. It is recognized primarily by crystal shape, prismatic habit (Figure 14.187), and association. Hardness and nearly square (diamond) cross sections help identify andalusite. A variety called chiastolite displays a “maltese cross” pattern that is diagnostic. Figures 14.188 and 14.189 show examples of chiastolite. Andalusite is sometimes confused with staurolite (which may have a similar habit and is also common in metapelites) or scapolite.
Physical Properties
| hardness | 7.5 |
| specific gravity | 3.18 |
| cleavage/fracture | rarely seen, good {110}, poor (100)/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | brown to red |
| streak | white |
Properties in Thin Section
Usually clear in thin section, andalusite may be weakly colored and pleochroic. Euhedral crystals with a square outline, sometimes showing penetration twins or a maltese cross pattern, are diagnostic. Andalusite is length fast and has a high 2V. Biaxial (-), α = 1.632 , β = 1.640, γ = 1.642, δ = 0.010, 2V = 75° to 85°.
Crystallography
Andalusite is orthorhombic, a = 7.78, b = 7.92, c = 5.57, Z = 4; space group \(P\dfrac{2_{1}}{n}\dfrac{2_{1}}{n}\dfrac{2_{1}}{m}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Stubby to elongate square prisms characterize andalusite. Individual crystals may be rounded. Massive and granular forms are also known.
Structure and Composition
Andalusite consists of chains of AlO6 octahedra parallel to the c-axis, linked by SiO4 tetrahedra and by AlO5 polyhedra. Andalusite is usually close to Al2SiO5 in composition. Small amounts of Mn, Fe, Cr, and Ti may be present.

Occurrence and Associations
Andalusite is a metamorphic mineral characteristic of relatively low pressures. It occurs in pelitic rocks, often associated with cordierite, sillimanite, kyanite, garnet, micas, and quartz. Figure 14.190 shows andalusite crystals in a muscovite schist from near Dublin, Ireland mica schist. The largest crystals are several centimeters long.
Varieties
Chiastolite is a variety of andalusite that has a square cross section (001) displaying a maltese cross pattern. The pattern results from carbonaceous impurities included during crystal growth.
Related Minerals
Andalusite has two polymorphs, sillimanite and kyanite.
Sillimanite Al2SiO5
Origin of Name
Named after Benjamin Silliman (1779–1864), a chemistry professor at Yale University.



Hand Specimen Identification
Sillimanite is found in high-grade pelites, in the form of fine needles (Figure 14.191), coarse blades (Figure 14.192), or prismatic acicular crystals (Figure 14.193) with square cross sections. The three figures here show examples. Aggregates may be masses or sprays. Sillimanite is occasionally confused with anthophyllite. Crystal form and habit, and occurrence in metapelitic rocks generally serve to identify this mineral.
Physical Properties
| hardness | 6 to 7 |
| specific gravity | 3.23 |
| cleavage/fracture | perfect but rarely seen (010)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | white to brown |
| streak | white |
Properties in Thin Section
Sillimanite typically forms needles, often in masses or mats, with square cross sections showing one good diagonal cleavage. It has high relief, small 2V, (+) optic sign, and is length slow. Biaxial (+), α = 1.658 , β = 1.662, γ = 1.680, δ = 0.022, 2V = 20° to 30°.
Crystallography
Sillimanite is orthorhombic, a = 7.44, b = 7.60, c = 5.75, Z = 4; space group \(P\dfrac{2_{1}}{b}\dfrac{2}{n}\dfrac{2}{m}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Long, slender prisms, needles, or fibers are common habits for sillimanite. Subparallel aggregates and sprays are typical. Fine-grained fibrous mats are also common.
Structure and Composition
Sillimanite‘s structure consists of chains of AlO6 octahedra, parallel to the c-axis,fa linked by SiO4 and AlO4 tetrahedra. Composition is always close to stochiometric Al2SiO5; small amounts of Fe may be present.


Occurrence and Associations
Sillimanite is the high-temperature Al2SiO5 polymorph, found in high-grade pelites associated with garnet, cordierite, spinel, hypersthene, orthoclase, biotite, and quartz. Often, individual sillimanite needles are hard to see but give a metamorphosed rock a foliated texture, like the example rocks in Figures 14.194 and 14.195. If you enlarge the photos, you can (just barely) make out the sillimanite needles.
Varieties
Fibrolite is the name given to fine-grained fibrous masses of sillimanite. Fibrolite is generally only visible in thin section.
Related Minerals
Sillimanite has two polymorphs: andalusite and kyanite.
14.1.5.5 Other Isolated Tetrahedral Silicates
Other Isolated Tetrahedral Silicates
staurolite Fe2Al9Si4O23(OH)
chloritoid (Fe,Mg)Al2SiO5(OH)2
titanite CaTiSiO5
topaz Al2SiO4(F,OH)2
zircon ZrSiO4
Besides those already listed, a number of other isolated tetrahedral silicates are common and important minerals. Although they have structures based on isolated SiO4 tetrahedra, they do not fit into any of the previously discussed structural groups.
Staurolite and chloritoid are important metamorphic minerals in rocks rich in Fe and Al. Titanite and zircon are common accessory minerals in silicic igneous rocks and in many metamorphic rocks. Topaz is most commonly found in pegmatites and hydrothermal veins associated with granites and other silicic igneous rocks.
Staurolite Fe2Al9Si4O23(OH)
Origin of Name
From the Greek word stauros, meaning “cross,” in reference to its cruciform twins.


Hand Specimen Identification
Staurolite is often easily recognized by its brown color, characteristic penetration twins, and prismatic crystals with a diamond-shaped cross section. These two photos show examples.
Staurolite is sometimes confused with andalusite, in part because of its typical brown color and occurrence in metapelites, but has different habit. Pyroxene, tourmaline, titanite, and amphibole may look superficially like staurolite.
Physical Properties
| hardness | 7 to 7.5 |
| specific gravity | 3.75 |
| cleavage/fracture | poor {010}/subconchoidal |
| luster/transparency | vitreous, sometimes resinous/translucent |
| color | brown to gray or black |
| streak | white |
Properties in Thin Section
Staurolite may be clear to yellow or light brown in thin section, often pleochroic. Birefringence is low; maximum colors are first-order yellow. Anhedral to euhedral porphyroblasts, often exhibiting a “sieve” structure due to quartz inclusions, or showing penetration twins, are common. Biaxial (+), α = 1.740 , β = 1.744, γ = 1.753, δ = 0.013, 2V = 80° to 88°.
Crystallography
Staurolite is monoclinic, a = 7.82, b = 16.52, c = 5.63, β = 90.0°, Z = 2; space group \(C\dfrac{2}{m}\); point group \(\dfrac{2}{m}\).
Habit
Staurolite is usually found as prismatic crystals, often flattened in one direction and having several terminating forms. Massive varieties are rare. Penetration twins are common, often resulting in perfect cruciform crosses, sometimes called fairy crosses (Figure 14.197, above).
Structure and Composition
Staurolite structure is closely related to that of kyanite. Layers of Al2SiO5, including AlO6 octahedra in chains, alternate with layers of Fe(OH)2. Pure end-member Fe-staurolite does not exist in nature; Mg is always present, replacing up to 35% of the Fe. Small amounts of Ti and Mn are generally present as well. Water content is slightly variable.

Occurrence and Associations
Staurolite is a metamorphic mineral common in medium- to high-grade metamorphic rocks. Associated minerals include kyanite, garnet, chloritoid, micas, and tourmaline. The rock seen in Figure 14.198 is a typical staurolite schist that contains coarse crystals of staurolite in a sea of sparkly mica.
Chloritoid (Fe,Mg)Al2SiO5(OH)2
Origin of Name
Named for its resemblance to chlorite.



Hand Specimen Identification
Green color, cleavage, occurrence in metapelites, and association with other pelitic minerals help identify chloritoid. In many metamorphic rocks it appears as small dark grains, green to black, or as patches in a micaceous matrix – and is hard to see. Thin sections may be required to distinguish it from chlorite, biotite, or stilpnomelane. Figure 14.199 shows a typical example. The photos in Figures 14.200 and 14.201 show exceptional coarser euhedral specimens.
Physical Properties
| hardness | 6.5 |
| specific gravity | 3.5 |
| cleavage/fracture | poor{110}/uneven |
| luster/transparency | pearly, sometimes vitreous/translucent |
| color | dark green or gray/black |
| streak | gray |
Properties in Thin Section
Chloritoid is typically colorless to green in thin section and may exhibit pleochroism in various shades of green, yellow, or blue. It has high relief and anomalous interference colors and is frequently twinned. Chlorite looks superficially like chloritoid but has significantly lower RI and relief and a smaller 2V. Biaxial (+), α = 1.715 , β = 1.720, γ = 1.725, δ = 0.010, 2V = 45° to 65°.
Crystallography
Chloritoid is monoclinic, a = 9.52, b = 5.47, c = 18.19, β = 101.65°, Z = 6; space group \(C\dfrac{2}{c}\); point group \(\dfrac{2}{m}\).
Habit
Coarse masses or thin scales are typical for chloritoid; individual crystals are rare. Tabular crystals are platy and foliated with common {001} twinning.
Structure and Composition
The chloritoid structure is layered. Alternating brucite-like and corundum-like layers, perpendicular to the c-axis, are linked by SiO4 tetrahedra and hydrogen bonds. Chloritoid is not a layered silicate, like chlorite, because the SiO4 tetrahedra do not share oxygen. Chloritoid is generally Fe-rich, but Fe:Mg ratios are variable; end members are not found in nature. Some Mn may be present.
Occurrence and Associations
Chloritoid is common in low- or medium-grade Fe-and Al-rich schists. Associated minerals include quartz, feldspars, muscovite, chlorite, staurolite, garnet, andalusite, and kyanite. In some rare high-pressure metamorphic rocks, it occurs with glaucophane and other blueschist minerals.
Varieties
Ottrelite is Mn-rich chloritoid; carboirite is Ge-containing chloritoid.
Related Minerals
Several different polytypes and polymorphs have been described.
Titanite (Sphene) CaTiSiO5
Origin of Name
This mineral’s name refers to its titanium content. Its older name, sphene, refers to its crystal (sphenoid) shape.


Hand Specimen Identification
Adamantine or vitreous luster, brown or green color, and diamond or wedge-shaped crystals help identify titanite. You can see the diagnostic shapes in some of the crystals in these two photographs.
In most rocks, titanite is so fine grained that it is hard to pick out without a thin section and microscope. Coarse crystals may occasionally be confused with staurolite and zircon, but titanite is softer; or with sphalerite, but titanite is harder.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 3.50 |
| cleavage/fracture | good prismatic {110}, poor (100)/uneven |
| luster/transparency | adamantine or vitreous/transparent to translucent |
| color | usually brown, less commonly gray-green, yellow-green, or black |
| streak | white |
Properties in Thin Section
Very high relief and birefringence and distinctive wedge- or diamond-shaped crystals characterize titanite. Biaxial (+), α = 1.86 , β = 1.93, γ = 2.10, δ = 0.15, 2V = 23° to 50°.
Crystallography
Titanite is monoclinic, a = 6.56, b = 8.72, c = 7.44, β = 119.72°, Z = 4; space group \(C\dfrac{2}{c}\); point group \(\dfrac{2}{m}\).
Habit
Sphenoidal crystals, tabular with a wedge or diamond shape in cross section, are typical. Less commonly, titanite is massive or lamellar. Titanite is normally fine grained but occasionally occurs as large crystals.
Structure and Composition
Titanite‘s structure contains TiO6 octahedra and SiO4 tetrahedra that share corners, forming distorted chains parallel to a. Ca is in 7-fold coordination, in large holes between the Ti- and Si-polyhedra. Many elements may substitute in titanite; the rare earth elements are especially important.
Occurrence and Associations
Titanite may be very fine grained and is an often overlooked, but common, widespread accessory mineral. In many rocks it is the only Ti mineral present. It is found in many igneous rocks, especially silicic to intermediate ones, and many metamorphic rocks. It also has been found in some limestones and a few rare clastic sediments. Associated minerals include just about all the important rock forming minerals, including pyroxene, amphibole, feldspar, and quartz.

Varieties
Greenovite (Figure 14.204) is the name given to red or pink titanite.
Related Minerals
Titanite is isostructural with tilasite, CaMg(AsO4)F; malayaite, CaSn(SiO4)O; and with fersmantite, (Ca,Na)4(Ti,Nb)2Si2O11(F,OH)2. Other related titanium minerals include perovskite, CaTiO3; benitoite, BaTiSi3O9; and neptunite, KNa2Li(Fe,Mn)2Ti2O(Si4O11)2.
Topaz Al2SiO4(F,OH)2
Origin of Name
Named after Topazion, an island in the Red Sea.



Hand Specimen Identification
Orthorhombic form, hardness (H=8), one good cleavage, color, and luster identify topaz. It may be confused with quartz but is orthorhombic, not hexagonal. The very rare mineral danburite, Ca(B2Si2O8), is similar to topaz in form and other properties, and sometimes cannot be distinguished without chemical analysis.

Topaz has many different appearances but when euhedral can appear as well-formed orthorhombic crystals. The clear crystals shown in Figure 14.205, and the yellow ones in Figures 14.206 and 14.207 are most typical. Colorful and gemmy topaz is especially valued by mineral collectors and gemologists –Figure 14.208 shows an example of pink gem topaz from Pakistan.
Physical Properties
| hardness | 8 |
| specific gravity | 3.5 to 3.6 |
| cleavage/fracture | one perfect (001)/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | generally colorless, but sometimes variable hues |
| streak | white |
Properties in Thin Section
Topaz is typically colorless in thin section and has low birefringence. It resembles quartz and apatite but has one perfect cleavage and higher relief than quartz, is biaxial, and is length slow. Biaxial (+), α = 1.61 , β = 1.61, γ = 1.62, δ = 0.01, 2V = 48° to 65°.
Crystallography
Topaz is orthorhombic, a = 4.65, b = 8.80, c = 8.40, Z = 4; space group \(P\dfrac{2_{1}}{b}\dfrac{2_{1}}{n}\dfrac{2_{1}}{m}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Typical topaz crystals are orthorhombic prisms, terminated by dipyramids and pinacoids in combination. Prism faces show striations. Cross sections may be square, rectangular, diamond shaped, or octagonal. Coarse- or fine-grained masses are also common.
Structure and Composition
The structure of topaz consists of chains, parallel to the c-axis, containing pairs of edge-sharing Al(OH,F)6 octahedra alternating with SiO4 tetrahedra. F and OH content are variable, but F:OH ratio is usually in excess of 6:1. No other significant substitutions are known.
Occurrence and Associations
Topaz is a late-stage igneous or hydrothermal mineral. It is an accessory in granite, rhyolite, and granitic pegmatites and may be found in contact aureoles adjacent to silicic plutons. It is often associated with lithium and tin mineralization. Associated minerals include quartz, feldspar, muscovite, tourmaline, fluorite, cassiterite, apatite, and beryl. It may be very fine-grained and easily overlooked in many kinds of rocks.
Related Minerals
Euclase, BeAl(SiO4)(OH), is isotypical with topaz.
Zircon ZrSiO4
Origin of Name
From the Persian zar (“gold”) and gun (“color”).



Hand Specimen Identification
Zircon can sometimes be identified by its hardness, brown to brown-red color, and tetragonal crystal shape. Tetragonal symmetry shows in Figures 14.209 and 14.210. But when the symmetry is not evident, like the specimens in Figure 14.211, zircon can be confused with other red-brown minerals such as titanite or rutile.
Physical Properties
| hardness | 7.5 |
| specific gravity | 4.68 |
| cleavage/fracture | poor (100), poor {101}/conchoidal |
| luster/transparency | adamantine, sometimes resinous/transparent to translucent |
| color | red or red-brown is typical, also gray, green, or colorless |
| streak | white |
Properties in Thin Section
In thin section, zircon is normally colorless but may be pale yellow or brown and faintly pleochroic. Grains are typically small, exhibiting very high relief and birefringence. Pleochroic halos around grains are due to decay of radioactive elements. Uniaxial (+), ω = 1.99, ε = 1.93, δ = 0.06.
Crystallography
Zircon is tetragonal, a = 6.59, c = 5.99, Z = 4; space group \(I\dfrac{4_{1}}{a}\dfrac{2}{m}\dfrac{2}{d}\); point group \(\dfrac{4}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Zircon typically is found as square prisms, pyramids/dipyramids, or as combinations of the two. Rounded grains are also common.
Structure and Composition
The zircon structure contains SiO4 tetrahedra sharing corners or edges with distorted cubic polyhedra containing Zr. Zircon is generally close to end-member composition, but frequently contains small amounts of Al, Fe, Mg, Ca, rare earths, and water.
Occurrence and Associations
Zircon is a common and widespread accessory mineral in igneous rocks, especially silicic ones. It is found in many metamorphic rocks and is common in sediments and sedimentary rocks. It is an important mineral for some kinds of radiometric dating. Grain size in rocks can be very small, so zircon is easily overlooked.
Varieties
Zircon is often metamict (structurally damaged by the decay of radioactive elements in its structure), causing variation in color and optical properties.
Related Minerals
Zircon has a number of isotypes, including thorite, (Th,U)(SiO4); xenotime, Y(PO4); and huttonite, ThSiO4. Baddeleyite, ZrO2, is another Zr-rich mineral.


