13.7.3: Single Chain Silicates (Pyroxenes and Pyroxenoids)
- Page ID
- 18366
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)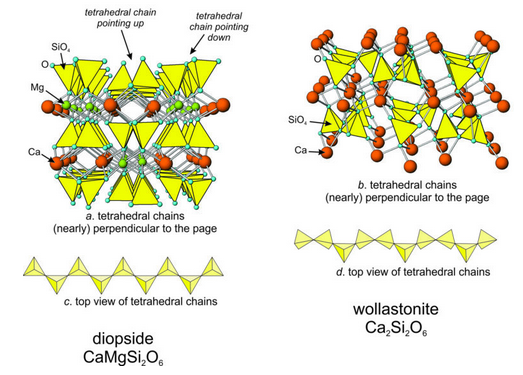
Figure 13.34 shows atomic arrangement in diopside, a pyroxene, and wollastonite, a pyroxenoid. In Chapter 6 we saw that pyroxenes and pyroxenoids contain chains of silica tetrahedra (see Figure 6.70). The chains are parallel to the c axes of the minerals In the Figure here (13.34), the view in drawings a (diopside) and b (wollastonite) is down the chain direction. Drawings c (diopside) and d (wollastonite) are top views of the chains.
Pyroxenes and pyroxenoids are both single-chain silicates. The main difference between them is subtle but can be seen by comparing Figures 13.34c and 13.34d. In pyroxenes the tetrahedra zigzag back and forth along the chain direction. In pyroxenoids, the pattern is more irregular. Pyroxenoids vary, but in wollastonite, the tetrahedral pattern repeats after three tetrahedra. As discussed in Chapter 6, pyroxenes may be orthorhombic or monoclinic. Twinning, both simple and complex, is common for both groups of minerals.
Pyroxenoids, which are triclinic, are much less common than pyroxenes. Although similar to pyroxenes, pyroxenoid atomic arrangements contain more large octahedral sites. The most common members of this group include wollastonite (Ca2Si2O6), rhodonite (Mn2Si2O6), bustamite (CaMnSi2O6), and pectolite (NaCa2Si3O8(OH)). Because pyroxenoids are generally rare and their structures are difficult to depict, we will focus on pyroxenes for the rest of this discussion.
In pyroxenes, tetrahedra making up a chain all point the same direction (either up or down in Figure 13.34a). And tetrahedra in adjacent chains point in opposite directions. In the diopside figure, for example, chains of tetrahedra pointing up have chains to their left or right that are pointing down. Similarly, chains pointing down have chains on either side pointing up.
The oxygen anions at the top of chains pointing up (and at the bottom chains pointing down) are called apical oxygens because they are at the apices of tetrahedra. These oxygen bond to octahedral cations (Ca2+ and Mg2+ in diopside) between chains, creating a three-dimensional structure. In single chain silicates, cations are exclusively tetrahedral or octahedral. Consequently, K+ and other large cations cannot be present.
Pyroxenes and pyroxenoids contain four octahedral sites between apical oxygen. Two of the sites, called the M2 sites (shown in orange in Figure 13.34a), are larger than the other two (the green M1 sites). In diopside and other calcic pyroxenes, the large M2 site contains Ca2+ and the M1 site contains smaller cations. These other cations are commonly Fe2+, Mg2+ or other cations of similar size and charge. The large M2 site in pyroxenes has a strong affinity for Ca2+, and the smaller site does not. Pyroxenes with M2 filled (or mostly filled) with Ca2+ are very stable compared with pyroxenes having a mix of different cations on that site. Consequently, a large miscibility gap (discussed in Chapter 6) separates calcic pyroxenes from those that are Ca-poor.
Besides the elements already discussed, pyroxenes may contain significant amounts of Na+, Mn2+, Ti4+, Fe3+, and Al3+. Some pyroxenes contain significant amounts of octahedral Al3+ in their M sites. If so, Al3+ also substitutes for Si4+ in tetrahedral sites so there is not too much cation charge. This is a coupled substitution, described as Al3+Al3+ = (Mg,Fe)2+Si4+ earlier in this chapter. We call it a tschermak substitution, named after 19th century Austrian mineralogist Gustav Tschermak. Other coupled substitutions are common in pyroxenes. For example, spodumene, LiAlSi2O6, is an important pyroxene in some pegmatites. It is related to diopside by the coupled substitution Li+Al3+ = Ca2+Mg2+.
Diopside, shown in Figure 13.35 below, has relatively simple chemistry. The only major cations it contains, besides Si4+, are Ca2+ and Mg2+. Other pyroxenes may contain many different elements. Perhaps the most common pyroxene species is augite (Figure 13.36), a dark green to black pyroxene that typifies mafic and some other igneous rocks. A simplified formula for augite is (Na,Ca)(Mg,Fe,Al,Ti)(Si,Al)2O6. The parentheses (from left to right) group elements that occupy the large octahedral site, the smaller octahedral site, and the tetrahedral site. Chapter 6 contains additional photos of pyroxenes: jadeite (Figure 6.73), spodumene (Figure 6.74), enstatite (Figure 6.76), diopside (Figure 6.77), and augite (Figure 6.78).




