7.4.6: Zeolites
- Page ID
- 18697
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
This photograph (Figure 7.56) shows orange chabazite crystals on top of heulandite. Both of these are zeolite minerals that occur most commonly in openings within basalt, and less commonly in metamorphic rocks or hydrothermal veins. This sample comes from a classic locality near Wasson Bluff, on the shore of the Bay of Fundy, Nova Scotia, where many outcrops of 200-million year old basalt contain cavities filled with spectacular mineral specimens.
Although zeolites are best known for their occurrences in vugs and other open cavities in volcanic rocks, large volumes are found in volcanic ashes and saline lake deposits. Zeolites also may be present as products of diagenesis or low-grade metamorphism in sediments and rocks.
| Zeolite Minerals | |
| natrolite analcime laumontite chabazite clinoptilolite heulandite stilbite sodalite |
Na2Al2Si3O10∙2H2O NaAlSi2O6·H2O CaAl2Si4O12∙4H2O CaAl2Si4O12∙6H2O (Na,K)Al2Si7O18∙6H2O CaAl2Si7O18∙6H2O CaAl2Si7O18∙7H2O Na3Al3Si3O12∙NaCl |
The zeolite group includes more than 40 minerals. They are, essentially, hydrated feldspars and are framework silicates containing open cavities that can hold loosely bonded large cations and water.
The compositions of zeolites seem highly variable, but follow certain patterns. The ratio (Al+Si):O is 1:2. In calcic zeolites, the ratio (Ca:Al) is always 1:2, and in alkali zeolites, the ratio (Na,K):Al is always 1:1. Zeolites and feldspathoids are closely related, but zeolites have more open structures and contain loosely bonded H2O compared with feldspathoids. Sodalite is sometimes grouped with the feldspathoids rather than the zeolites, but it has a zeolite-type structure and contains loosely bonded NaCl. Analcime, NaAlSi2O6∙H2O, is also sometimes considered a zeolite, but is closer to leucite and other feldspathoids in atomic structure.
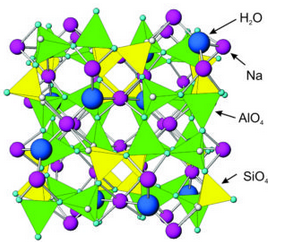
Figure 7.57 shows the atomic arrangement in analcime – it is similar in most ways to all zeolites. All zeolites contain tetrahedra with aluminum and silicon cations surrounded by four oxygen anions. Corners of the tetrahedra join to other corners.
Analcime and other zeolites contain holes and channels between tetrahedra that can hold or transmit large ions, such as sodium, calcium, potassium, H2O, or other molecules. This property sets them apart from most other minerals. Different zeolites have different-sized openings, and in some zeolites the openings connect to form channels. Because of the cavities and channels, zeolites have many industrial applications.
Industrial Zeolites

What do water softeners, fish tanks, kitty litter, and people with radioactive poisoning have in common? The answer is zeolites.
Zeolites contain water that can be driven off by heat with the basic structure left intact. Removal of water leaves voids that can be filled, making them useful for many purposes, including ion exchange, filtering, odor removal, chemical sieving, and gas absorption. The best-known use for zeolites is in water softeners. Because calcium in water causes it to be hard, suppressing the effects of soap, forming scale, or creating other problems, it is often filtered through zeolite. Zeolite charged with sodium ions (generally from dissolved salt) allows water to pass through its structure while exchanging calcium for sodium, thus making the water less hard. This process is reversible, so the zeolite can be flushed and used again.
In a similar way, zeolites can absorb unwanted chemicals, including toxins. For example, zeolites may be components of kitty litter because they absorb cat urine, and zeolites are added to livestock feed to absorb toxins that are damaging or fatal to animals. Additionally, people who have been exposed to radioactive elements ingest zeolites to help rid their system of those dangerous elements, and aquarium hobbyists use zeolites to remove ammonia and other toxins from fish tanks. In the highly-developed countries of the world, most municipal water is processed through zeolites to remove large ions and organic contaminants before public consumption. Manufacturing industries also use zeolites as drying agents, catalysts, and as washing materials.
Synthetic zeolites are easily grown in the laboratory, and scientists have synthesized more than 150 varieties. For many applications, synthetic zeolites have advantages over their natural cousins because they can be manufactured to be pure and homogeneous, and to have unique properties not found in nature.


