7.1.1: Two Kinds of Weathering
- Page ID
- 18686
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Figure 7.2 shows the two parallel processes that may occur during weathering. An original source rock (igneous, metamorphic, or sedimentary) is exposed to forces that cause weathering. The weathering forces may be mechanical (due, for example, to forces applied by water, wind, or glaciers) or chemical (for example, dissolution by water). Often the two kinds of weathering work together. And, these processes are selective. Some minerals dissolve or react and disappear faster than other minerals. Some rocks are harder and do not break apart as easily as other rocks. Over long times – geological times – chemical weathering has a much greater effect than mechanical weathering. Even apparently dry climates have enough water to promote chemical weathering on exposed surfaces, although the weathering rate may be slow.
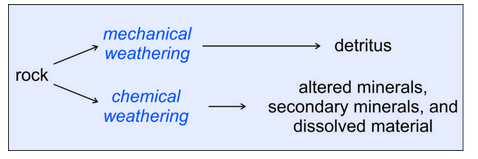
Mechanical weathering breaks large or solid material into smaller pieces. The products produced vary greatly; we call them collectively clasts (from klastos, the Greek word meaning “broken”). Clastic material, also called detritus or detrital material, may be fine grains of individual minerals, or it may be lithic fragments (rock fragments) composed of multiple minerals.

The photo seen here (Figure 7.3) shows boulders on a talus slope that were produced by mechanical weathering (mostly freezing and thawing) in Yellowstone National Park. Freezing, thawing, and the action of ice created large blocky pieces of what was originally solid bedrock. Even apparently solid granites or other rocks can be broken apart this way. Talus slopes are examples of very coarse sediment. More commonly, mechanical weathering produces smaller rock fragments, or sand, or silt composed of individual mineral grains.

After chemical weathering, leftover rock may have a dissolved or eroded appearance, such as the sandstone seen in Figure 7.4. This sandstone has weathered to obtain a honeycomb texture, typical of sandstone in which the cementation of grains is not uniform.
Weathering textures are not unique to sandstone. After chemical weathering, outcrops of many sorts often become rounded or pitted. The limestone outcrop shown in Figure 7.5, for example, shows signs of dissolution and chemical weathering. The surfaces are a dull chalky white and the corners are all rounded. Figure 4.24 (Chapter 4) shows another example of chemical weathering, a weathering rind on sandstone.

Minor chemical weathering can cause minerals to alter, perhaps to discolor or rust. More intense weathering may cause some minerals to disappear. They may dissolve completely in water and be carried away in a hydrolysate (water containing dissolved ions). Often, minerals react to produce secondary minerals that were not present before weathering. Reactions that produce secondary minerals most commonly involve water reacting with previously existing minerals. For example, during chemical weathering, feldspars (common in many igneous rocks) react with water to produce clays and dissolved elements. We call such reactions hydrolysis reactions. Secondary minerals may also form by oxidation reactions when primary minerals react with oxygen in air or water. For example, oxidation of iron-rich olivine or pyroxene commonly produces hematite (Fe2O3).
Mineral matter remaining after chemical weathering often includes original mineral grains that did not decompose. We sometimes call these minerals the residual minerals, or the resistate, because the minerals resisted weathering. Typical resistate minerals include quartz, clay, K-feldspar, garnet, zircon, rutile, or magnetite. After the more easily decomposed minerals break down and disappear, the resistate minerals remain to become sediment.


