6.4.10: Olivine Group Minerals
- Page ID
- 18940
| Olivine Group Minerals (Mg,Fe,Ca,Mn)SiO4 |
|
| Olivine end members | |
| forsterite fayalite tephroite calcio-olivine |
Mg2SiO4 Fe2SiO4 Mn2SiO4 Ca2SiO4 |
| Other olivine group minerals | |
| larnite monticellite |
Ca2SiO4 CaMgSiO4 |
| Related minerals | |
| zircon topaz |
ZrSiO4 Al2SiO4(F,OH)2 |
Olivine group minerals, belonging to the isolated tetrahedra silicate subclass, all have similar atomic arrangements. By far, the most important mineral of this group is called olivine. In contrast with some of the other silicates previously discussed, olivine chemistry is quite simple. Its general formula is (Mg,Fe,Ca,Mn)2SiO4 but often we omit Mn and Ca because they are normally minor components. The most important end members are listed in the blue box.
Besides olivine, other minerals of the group include larnite, a polymorph of calcio-olivine, and monticellite. Both are rare. Zircon and topaz, have different atomic arrangements than the olivine minerals and so are not members of the group. They are, however, other important isolated tetrahedra silicates and so are listed here.
Natural olivines are generally solid solutions of fayalite and forsterite with only minor tephroite and calcio-olivine. We usually use abbreviations and subscripts to indicate olivine compositions. Fo88Fa09Te02La01, for example, refers to an olivine of composition (Mg0.88Fe0.09Mn0.02Ca0.01)2SiO4.
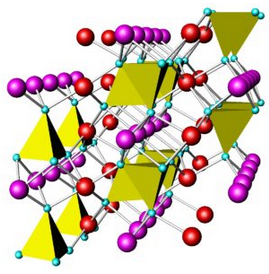
In olivine group minerals, SiO4 tetrahedra do not share oxygen but, instead, are linked via bonds to other cations. Figure 6.94 shows an example. The red and purple spheres are mostly Fe and Mg in common olivine. In monticellite, the red spheres are Ca and the purple spheres are Mg. Because the silicon tetrahedra are isolated, bond strength is about the same in all directions. Consequently, olivine has no direction of good cleavage.
Olivine is primarily an igneous mineral, crystallizing from high-temperature magmas. Its two most important end members, forsterite and fayalite, melt at different temperatures, and olivine’s crystallization behavior is similar to that of plagioclase.
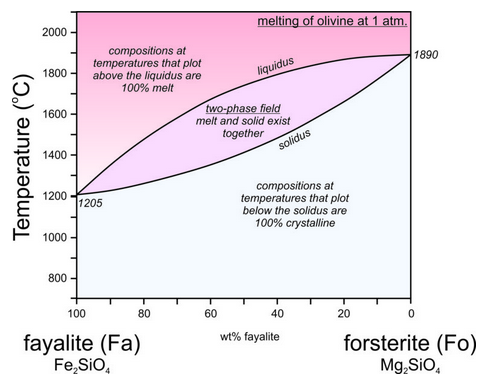
Figure 6.95 is a phase diagram that describes crystallization of forsterite-fayalite olivines, and the two-phase field between the liquidus and solidus. Compare this with Figure 6.54 (plagioclase phase diagram); they are nearly identical. Partial crystallization of olivine-rich melts leads to crystals that are more Mg-rich than the parent melt, and partial melting of olivine crystals leads to melts that are more Fe-rich than the parent crystals. If the melt and crystals become separated, the Fe-rich melt will move upwards and carry iron from depth toward Earth’s surface. Magnesium will be left behind. This is one reason why Earth’s crust is more Fe-rich than the mantle.


Olivine occurs predominantly in mafic and ultramafic igneous rocks. Its very mafic composition restricts its occurrence, and other mafic minerals containing the same elements with additional silicon are more abundant. In some basalts Mg-rich olivine forms large green phenocrysts in a fine-grained plagioclase-pyroxene groundmass; Figure 6.96 shows an example from the Canary Islands.
Mg-rich olivine is never found in felsic rocks, but Fe-rich olivine is occasionally found in some granites. Olivine occurs in some metamorphic rocks, including marble and metamorphosed igneous rocks. Olivine also is found in mantle xenoliths – samples of the mantle that have been carried to the surface by magmas. The photo in Figure 6.97 is dark basalt that contains a mostly green xenolith. The green colored crystals in the xenolith are olivine. Dark brown orthopyroxene is also present, but in lesser amounts.


