1.4.2: An Example- Pyroxenes
- Page ID
- 18285
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Let’s consider the pyroxene group of minerals. All pyroxenes have the general formula ABT2O6. The A and B atoms may be the same or different but are typically Fe, Mg, Ca, Mn, and sometimes Na. T atoms are mostly Si but sometimes up to half Al. The chart below shows how pyroxenes are classified into class, subclass, group, series or subgroup, and species.
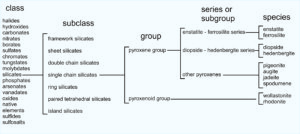
1.34 Classification of pyroxene and pyroxenoid group minerals

1.35 Diopside, garnet, and clinochlore
As seen in the chart, pyroxenes belong to the silicate class and single chain silicate subclass. Single chain silicates include minerals of the pyroxene group and minerals of the pyroxenoid group. The groups contain specific species such as enstatite (Mg2Si2O6), ferrosilite (Fe2Si2O6), diopside (CaMgSi2O6), hedenbergite (CaFeSi2O6), and others listed in the far right column of the chart above.
The pyroxene group also contains series, for example the diopside-hedenbergite series, and the enstatite-ferrosilite series. Series define a range of possible mineral compositions between two mineral species. Thus, from general to specific, pyroxenes belong to a mineral class, subclass, group, series, and then are identified as individual species. These relationships hold true for other mineral groups as well. The photo in Figure 1.35 shows light green diopside (a pyroxene) with red garnet and dark purple clinochlore in the background.
Although not shown in the chart above, we divide individual mineral species into varieties based on specific characteristics such as color or crystal shapes. For example, varieties of diopside include chrome-diopside (the emerald green chrome containing variety seen in Figure 1.36), dekalbite (diopside that contains no impurities at all), malacolite (a light colored or white variety that is usually fluorescent).
● Box 1-2 Chemical Formulas of MineralsThroughout this book, we follow standard chemical conventions when we write mineral formulas. We list elements with subscripts to indicate the relative numbers of atoms present. We list cations (positively charged ions) before anions (negatively charged ions) and molecular anionic species, with the largest cations coming first. The following are some examples of formulas following these rules:
Subscripts outside parentheses apply to everything within if no commas are present. The formula unit of marialite, for example, indicates that 4 Na, 3 Al, 9 Si, 24 O, and 1 Cl are in one formula of marialite. Commas show an either-or situation. In one formula of clinohumite, for example, there are two atoms of OH or of F, or of the two combined. If we had omitted the comma, it would mean that there were both 2 OH and 2 F per formula. Elements separated by commas, then, can be thought of as substituting for each other. For example, montmorillonite may contain either Al or Mg, or both; olivine may contain either Fe or Mg, or both. Parentheses surround anionic groups such as (SiO4) or (CO3) when it helps with clarity. In clinohumite, parentheses around (SiO4) emphasize clinohumite’s chemical similarity to forsterite and other olivines, all of which have (SiO4) in their formulas. In montmorillonite, the (Si4O10) is in parentheses to emphasize that the structure is that of a sheet silicate, many of which have (Si4O10) in their formula. Loosely bonded interstitial components (such as Cl in marialite, or OH and F in clinohumite) are on the right in the formulas. We indicate loosely bonded H2O, often called nonstructural water, by a dot preceding nH2O at the far right in a formula. The n (instead of an integer) in the formula for “clay” means that an unknown or variable amount of nonstructural water is present. Natrolite, a zeolite, has H2O in holes in its structure. When completely hydrated, there are two moles of H2O for each Na2Al2Si3O10 formula unit. When useful, we use superscripts to show ionic charge: (OH)– is the hydroxyl radical, which has a charge of -1. Similarly (SiO4)4- indicates an Si atom bonded to 4 O, with a net charge of -4. Sometimes showing coordination number (the number of bonds to an atom) is useful. We do this with superscript roman numerals; they are discussed later. |
●Figure CreditsUncredited graphics/photos came from the authors and other primary contributors to this book. 1.1 Blue cavansite on top of silvery heulandite, Géry Parent, Wikimedia Commons Video 1-1 What is a Mineral? Keith Purtirka, YouTube |



