4.7: Other Important Rock Forming Minerals
- Page ID
- 22618
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Calcite
We’ve made it through the formation of nine of “The Big Ten” minerals. At this point, you’re probably wondering, “what about calcite? As the final ‘Big Ten’ mineral, how does calcite form?” Calcite formation is most typically associated with sedimentary processes. Let’s take a look at calcite and some of the other “non-Big Ten” minerals that are important in Historical Geology.
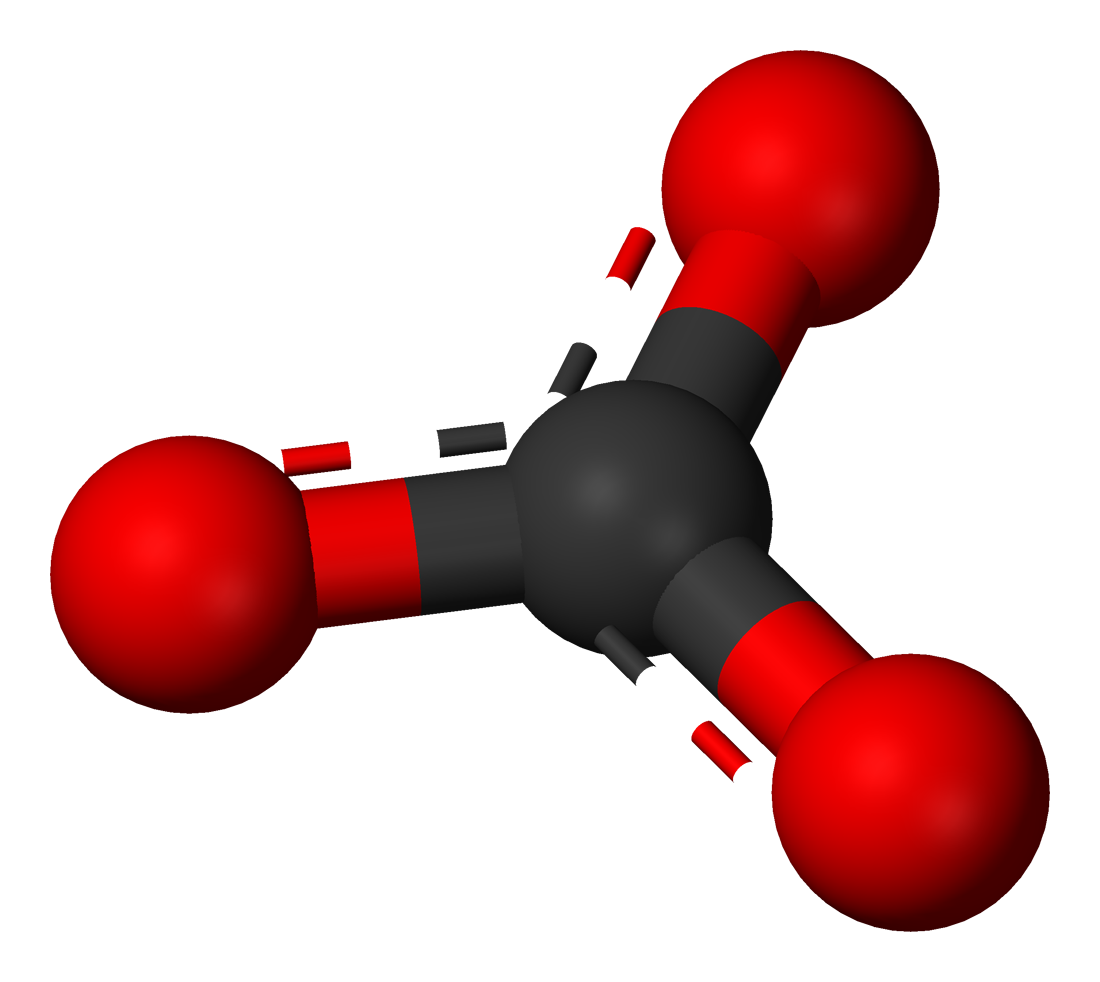
Calcite is a “non-silicate” mineral (see the pie chart of the minerals of the crust). Calcite belongs to another family, or group of minerals, called the carbonates. The basic chemical and crystalline foundation for calcite and other minerals of the carbonate group is the complex carbonate ion \(\ce{(CO3)^{-2}}\) where one carbon cation is surrounded by three oxygen anions in a triangular pattern.
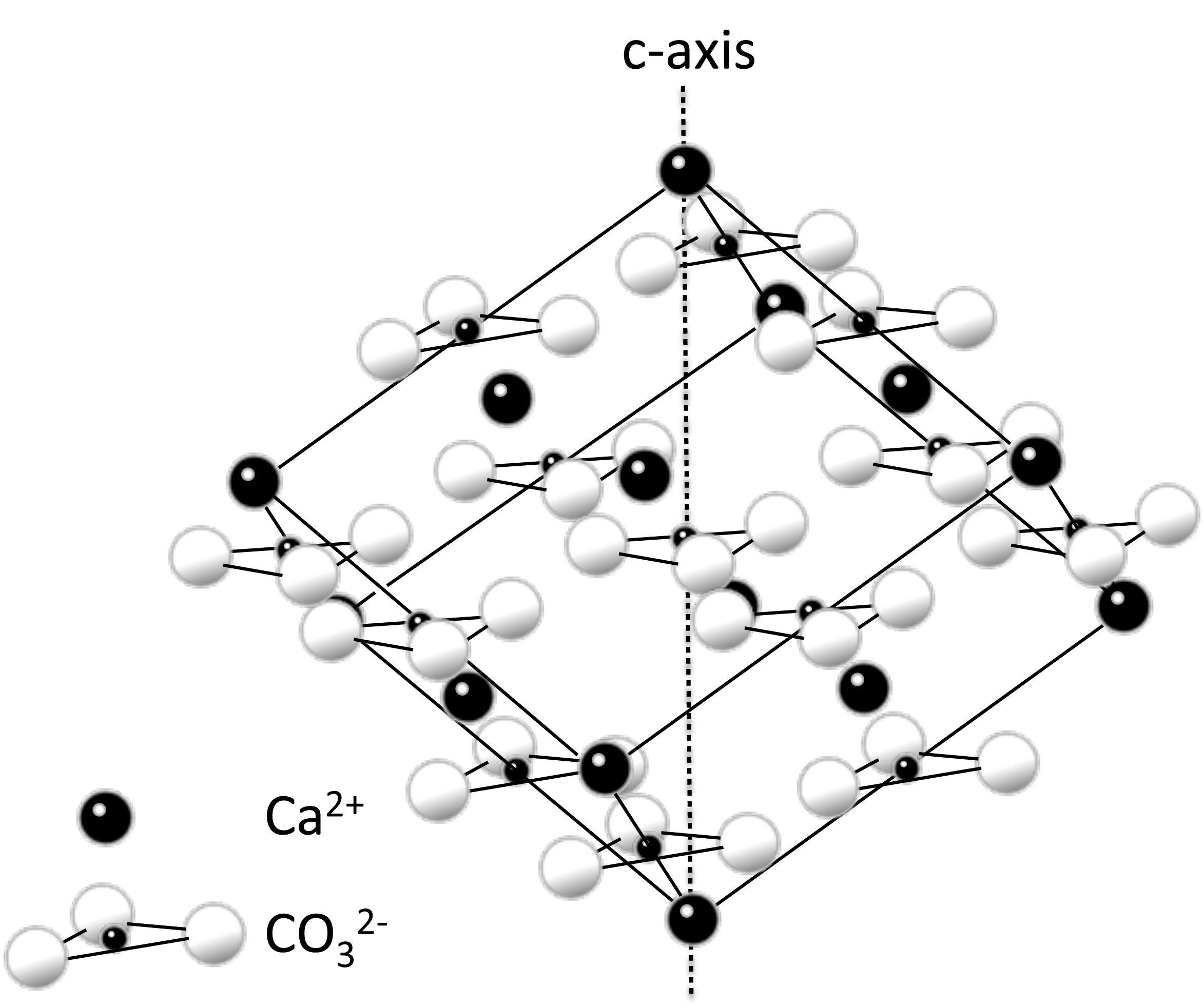
Calcite forms by the ionic bonding of the complex carbonate ion with a calcium anion. The diagram above represents one unit cell of the mineral calcite.
Crystallization of Calcite
Calcite can form by:
1. Biomineralization, where organisms like clams and corals extract calcium and carbonate ions from seawater or fresh water and then combine them inside their tissues to form their skeletal material.
2. Precipitation, where calcium and carbonate ions combine to precipitate micro-crystals of calcite from supersaturated seawater or circulating groundwater.
3. Evaporation, where inland lakes and epicontinental seas become isolated and slowly dry up leaving crystalline calcite plus other minerals.
4. In rare circumstances, calcite can occur through igneous processes.
One of the most common sedimentary rocks found on Earth is limestone. Most limestone is biogenic in origin, meaning, it is formed through the lithification of the skeletal remains of organisms. These organisms, both macroscopic and microscopic, have shells or exoskeletons composed of calcite. When these organisms die, their remains accumulate and their soft body parts are scavenged or decompose. The remaining calcite hard parts become trapped in layers of sediment and eventually are cemented together by additional calcite that precipitates in the pore spaces between the skeletal remains. The resulting sedimentary rock is called fossil limestone. This accounts for the majority of calcite formation on Earth. Other processes mentioned above require special environmental conditions to allow for the formation of calcite.
Dolomite is another common carbonate mineral closely related to calcite often occurring in similar environments of formation, making it another important mineral in the study of Historical Geology. Dolomite has many similar characteristics to calcite including its chemical formula \(\ce{CaMg(CO3)2}\).
Clay Minerals
You might be surprised to learn that clay is a mineral – and not just one mineral – but a whole group of minerals. Clay minerals are also silicate minerals (see previous pie chart) that form through the processes involved with weathering of pre-existing silicate minerals in the presence of weak acid and water. As rain forms in the atmosphere, it combines with carbon dioxide to form a weak acid called carbonic acid with the chemical formula, \(\ce{H2CO3}\). This weak acid rains down on Earth’s surface and immediately attacks minerals exposed at the surface. The pre-existing minerals become altered in their chemical composition through a reaction called hydrolysis. Hydrolysis is a type of chemical weathering that forms a wide range of clay minerals.

Clay minerals are sheet silicates similar to micas. Their chemical formula is dependent upon the minerals from which they were derived however all clay minerals contain a great deal of aluminum and silica plus varying amounts of water. Individual clay crystals tend to be very small, and often cannot be identified without a microscope or other equipment such as a scanning electron microscope (see photo below). Clays are important to the study of Historical Geology because they are the main components of fine-grained sediment (mud) and form another very common sedimentary rock, shale. The following is a hydrolysis reaction that occurs when silicate minerals encounter carbonic acid to produce clay and other ions:
\[\text{Silicate mineral }+ \text{ carbonic acid } \ce{(H2CO3)} + \ce{H2O} \rightarrow \text{ clay }+ \text{ metal cations } (\ce{Fe^{+2}, Mg^{+2}, Ca^{+2}, Na^+,} etc.) + \text{ bicarbonate anions } \ce{(HCO3)^{-1}} + \text{ silica } \ce{(SiO2)} \nonumber\]
Other Rock-Forming Silicates
The silicate minerals that fall into the group of “other” on the pie chart above (link to it) are largely formed through metamorphic processes. Metamorphism most commonly occurs deep within the Earth’s crust where rocks are buried and experience intense changes in temperature and pressure during periods of mountain building (orogeny). Some of the pre-existing minerals found within these deeply buried rocks can change in physical form and chemical composition as they recrystallize in response to these altered conditions.
One common metamorphic mineral that most students are familiar with is garnet. You might be surprised to learn that garnet is not just one mineral but also a part of a larger group. The most common garnet minerals are dark red in color (like the birthstone) and have the general chemical formula of:
\[\bf{\ce{(Ca, Mg, Fe)Al2(SiO4)3}}\nonumber\]
Garnets are largely formed through the metamorphism of clay minerals which are rich in aluminum and silica. These clay minerals would be found in the common sedimentary rock shale that is subjected to varying degrees of pressure and temperature changes during the collision of tectonic plates and the formation of mountains.
| Mineral Groups | Common Rock Forming Mineral |
|---|---|
| Silicates | Olivine Pyroxene (Augite) Amphibole (Hornblende) Biotite Muscovite Calcium-rich plagioclase feldspar (anorthite) Sodium-rich plagioclase feldspar (albite) Potassium-rich Feldspar (orthoclase) Quartz Clay Garnet |
| Carbonates | Calcite, Dolomite |
| Oxides | Hematite, Magnetite |
| Halides | Halite |
| Sulfides | Pyrite, Chalcopyrite, Galena, Sphalerite |
| Sulphates | Gypsum |
| Phosphates | Apatite |
| Native Elements | Gold, silver, copper |
Oxides, Halides, and Sulfides
After carbonates, the next most common non-silicate minerals are the oxides, halides, and sulfides.
Oxides
Oxides consist of metal ions covalently bonded with oxygen. Iron oxides are important for producing iron ore deposits from which we extract metallic iron. The formation of Earth’s major iron ore deposits are extremely important in studying Earth’s history. Prior to about two billion years ago (2 Ga), the early oceans were saturated with soluble iron due to the lack of free oxygen. So much iron existed in the water that our early oceans were thought to have been green in color from the dissolved iron. As oxygen levels slowly increased in the atmosphere and dissolved in the oceans, the oxygen immediately bonded with the iron forming the common ore minerals of hematite (\(\ce{Fe2O3}\)) and magnetite (\(\ce{Fe3O4}\)). (See the case study on the Great Oxidation Event).
The most familiar oxide of iron is rust, which is a combination of iron oxides (\(\ce{Fe2O3}\)) and hydrated oxides. Hydrated oxides form when iron is exposed to oxygen and water. The red color that we often see in soil and rock is usually due to the presence of iron oxides. For example, the red sandstone cliffs in Zion National Park and throughout Southern Utah consist of white or colorless grains of quartz coated with iron oxide which serves as cement, holding the grains together.

Halides
The halide minerals, also important to the study of historical geology contain the element chlorine ionically bonded with sodium or other cations. These minerals include halite or sodium chloride (NaCl; common table salt) and sylvite or potassium chloride (KCl), commonly used in cooking as a salt substitute.
Halide minerals are extremely important to the study of Earth’s history because their occurrence indicates a period of evaporation of sea water or other isolated bodies of water. A well-known example of modern halide mineral deposits created by evaporation is the Bonneville Salt Flats, located west of the Great Salt Lake in Utah (need a good pic). Watch the following video on extraction of rock salt from the Cayuga Salt Mine in central New York state. The halite mineral of the rock salt represents evaporation of a great inland sea that existed some 385 million years ago in the Great Lakes region.
Watch the following video where at 2300 feet underground, the Cayuga Mine in Lansing, NY, processes approximately 10,000 tons of road salt daily that is shipped to more than 1,500 locations throughout the northeast United States.
Sulfides
Many important metal ores are sulfides, in which metals are bonded to sulfur. Significant examples include: pyrite (iron sulfide, sometimes called “fool’s gold”), and chalcopyrite (iron-copper sulfide), galena (lead sulfide), sphalerite (zinc sulfide). Sulfides are well known for being important ore minerals. For example, galena is the main source of lead, sphalerite is the main source of zinc, and chalcopyrite is the main copper ore mineral.
The iron sulfide mineral pyrite is of particular importance to Historical Geology. Pyrite is unstable in the presence of oxygen and will readily chemically decompose to form stable iron oxide. However, pyrite occurs in some of Earth’s early (older than 2.0 Ga) sedimentary rocks. Sediments are formed from the weathering of pre-existing rock exposed on Earth’s surface. Pyrite, existing as sedimentary clasts, or pieces, within these ancient rocks, indicates that surface conditions must have been lacking oxygen to allow the pyrite to remain unaltered and ultimately preserved.
Sulfate Minerals
Sulfate minerals contain a metal ion, such as calcium, bonded to a sulfate ion. The sulfate ion is a combination of sulfur and oxygen \(\ce{(SO4)^{-2}}\). The sulfate mineral gypsum \(\ce{(CaSO4 \cdot 2H2O)}\) is important to Earth’s history because it indicates formation from evaporating water and usually contains water molecules in its crystalline structure.
Phosphate Minerals
Phosphate minerals contain the complex cation \(\ce{(PO4)^{-2}}\) combined with various anions and cations. The best known and important phosphate mineral to the study of historical geology is apatite, \(\ce{Ca5(PO4)3(F,Cl,OH)}\). Variations of this mineral are found in teeth and bones.
Native Elements
Native element minerals, usually metals, occur in nature in a pure or nearly pure state. Gold, silver and copper are examples of common native element minerals. Carbon is often found as a native element in minerals such as graphite and diamond.



