8.4: Fossil Fuels
- Page ID
- 25541
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There are several types of fossil fuels, but all of them involve the storage of organic matter in sediments or sedimentary rocks. All fossil fuels are rich in carbon and almost all of that carbon ultimately originates from CO2 taken out of the atmosphere millions of years ago during photosynthesis. That process, driven by solar energy, involves reduction (the opposite of oxidation) of the carbon, resulting in it being combined with hydrogen instead of oxygen. The resulting “organic matter” is made up of complex and varied carbohydrate molecules.
Most of the organic matter produced in this way is oxidized back to CO2 relatively quickly once the organism dies (within weeks to decades in most cases), but any of it that gets isolated from the oxygen of the atmosphere, for example deep in the ocean or in a stagnant bog, may last long enough to be buried by sediments and, if so, may be preserved for tens to hundreds of millions of years. Under natural conditions, that means it will be stored until those rocks are eventually exposed at surface and weathered.
In this section we’ll discuss the origins and extraction of the important fossils fuels, including coal, oil and gas. Coal, the first fossil fuel to be widely used, forms mostly on land in swampy areas adjacent to rivers and deltas in areas with humid tropical to temperate climates. The vigorous growth of vegetation leads to an abundance of organic matter that accumulates within the stagnant water of swamps, ponds and lakes with little circulation, and thus does not decay and oxidize. This situation, where the dead organic matter is submerged in oxygen-poor water, must be maintained for centuries to millennia in order for enough material to accumulate to form a thick layer (Figure 8.4.1a). At some point the swamp deposit is covered with more sediment—typically because a river changes its course or overtops its bank (Figure 8.4.1b). As more sediments are added the organic matter starts to become compressed and heated. Low grade lignite coal will form at depths between a few 100 m and 1500 m and temperatures up to about 50˚ C (Figure 8.4.1c). At between 1000 to 5000 m and temperatures up to 150˚ C m bituminous coal will form (Figure 8.4.1d). At depths beyond 5000 m and temperatures over 150˚ C anthracite coal will form.
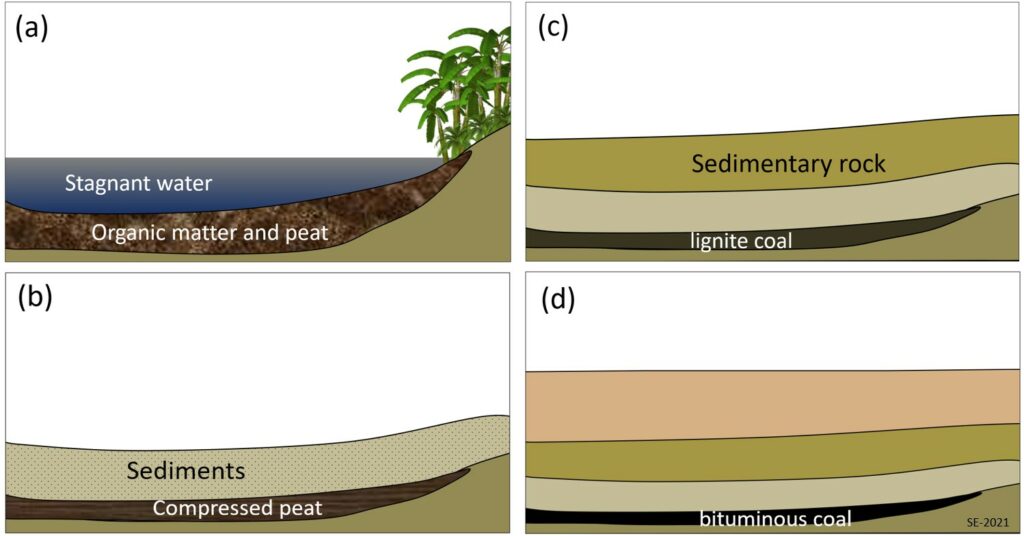
During the process of converting organic matter to coal some methane is produced and it is stored within the pores of the coal. When coal is mined methane is released into the mine where it can become a serious explosion hazard. Modern coal-mining machines have methane detectors on them and will actually stop operating if the methane levels are dangerous. It is possible to extract the methane from coal beds without mining the coal and the gas recovered in this way is known as coal bed methane.
While almost all coal is formed on land from terrestrial vegetation, most oil and gas is derived primarily from marine microorganisms that accumulate within sea-floor sediments. In areas where marine productivity is high dead organic matter is delivered to the seafloor fast enough that at least some of it escapes being oxidized. This material accumulates in the muddy sediments and then those get buried to significant depth beneath other sediments.
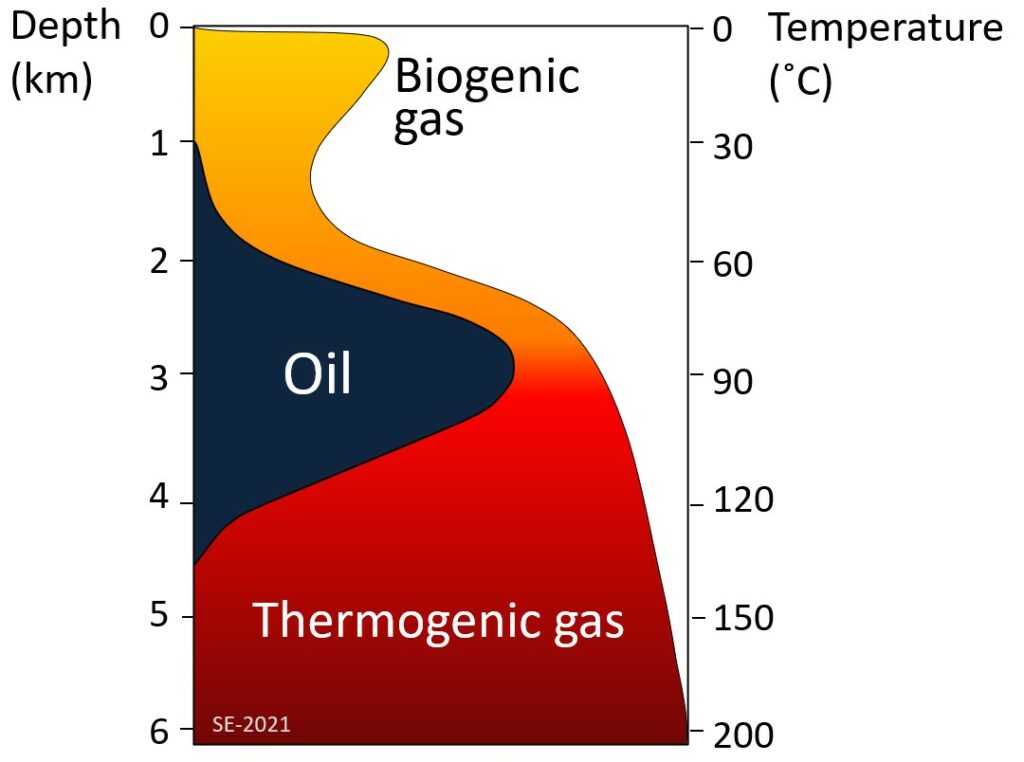
As the depth of burial increases so does the temperature—due to the geothermal gradient—and gradually the organic matter within the sediments gets converted to hydrocarbons (Figure 8.4.2). The first stage is the biological production (involving anaerobic bacteria) of methane. Most of this escapes back to surface, but some is trapped in methane hydrates near to the sea floor. At depths beyond about 2 km, and at temperatures ranging from 60 to 120˚ C, the organic matter is converted by chemical processes to oil. This depth and temperature range is known as the oil window. Beyond 120˚ C most of the organic matter is chemically converted to methane (i.e., natural gas).
The rock within which the formation of gas and oil takes place is known to petroleum geologists as the source rock. This rock is typically rich in organic matter, and a good example would be a black shale. Both liquid oil and gaseous methane are lighter than water, so as liquids and gases are formed they tend to move slowly towards surface, out of the source rock and into reservoir rocks. Reservoir rocks are typically relatively porous and permeable rocks such as sandstone or fractured limestone, because that allows migration of the fluids from the source rocks, and also facilitates recovery of the oil or gas. In some cases, the liquids and gases make it all the way to surface, where they are oxidized and the carbon is returned naturally to the atmosphere, but in others they are contained by overlying impermeable rocks (a.k.a. “cap rock”, e.g., mudrock) in situations where anticlines, faults, stratigraphy changes and reefs or salt domes create traps (Figure 8.4.3).

The liquids and gases that are trapped within reservoirs will become separated into layers based on their density, with gas rising to the top, then oil, and water underneath. The proportions of oil and gas will depend primarily on the temperature in the source rocks. Some petroleum fields, such as many of those in Alberta, are dominated by oil, while others, notably those in northeastern BC, are dominated by gas.
In general petroleum fields are not visible from surface, and their discovery involves the search for structures in the sub-surface that have the potential to form traps. Seismic surveys are the most commonly used tool for early-stage petroleum exploration, as they can reveal important information about the stratigraphy and structural geology of sub-surface sedimentary rocks. An example from the Gulf of Mexico south of Texas is shown on Figure 8.4.4. In this area a thick evaporite deposit (“salt”) has formed domes because salt is lighter than other sediments and tends to rise slowly towards surface, and this has created traps. The sequence of deformed rocks is capped with a layer of undeformed rock.

The type of oil and gas reservoirs illustrated in Figures 8.4.3 and 8.4.4 are described as conventional reserves. Some unconventional types of oil and gas include oil sands, shale gas and coal-bed methane.
Oil sands are important because the reserves in Alberta are so large (the largest single reserve of oil in the world), but they are very controversial from an environmental and social perspective. They are “unconventional” because the oil is exposed at surface and is highly viscous because of microbial changes that have taken place at surface. The hydrocarbons that form this reserve originated in deeply buried Paleozoic rocks adjacent to the Rocky Mountains and migrated up and towards the east (Figure 8.4.5).
The oil sands are controversial primarily because of the environmental cost of their extraction. Since the oil is so viscous, it requires heat energy to make it sufficiently liquid to process. This energy comes from gas; approximately 25 m3 of gas is used to produce 0.16 m3 (~one barrel) of oil. (That’s bad, but not quite as bad as it sounds, as the energy equivalent of the required gas is about 20% of the energy embodied in the produced oil.) The other environmental cost of oil sands production is the devastation of vast areas of land where strip-mining is taking place, and the unavoidable release of contaminants into the groundwater and rivers of the region.
At present most oil sand recovery is achieved by mining the sand and processing it on site. Exploitation of oil sand that is not exposed at surface depends on in situ processes, an example being the injection of steam into the oil-sand layer to reduce the viscosity of the oil so that it can be pumped to surface.

Shale gas is gas that is trapped within rock that is too impermeable for the gas to escape under normal conditions and can only be extracted by fracturing the reservoir rock using water and chemicals under extremely high pressure. This procedure is known as hydraulic fracturing or fracking. Fracking is controversial because of the volume of water used, and because the fracking companies are not required to disclose the nature of the chemicals used. Although fracking is typically done at significant depths there is always the risk that overlying water-supply aquifers could be contaminated (Figure 8.4.6). Fracking also induces low-level earthquakes, which do have the potential to cause damage.
It is important for us to understand the origins and exploitation of fossil fuels, but it is equally important to recognize that the Earth can no longer sustain the current rate of fossil fuel use, or in fact any use at all. If we want to avoid catastrophic climate change we need to reduce the use and production of fossil fuels quickly—eventually to zero, and that means there is no point in searching for new fossil fuel resources, or in further developing known resources.
At the United Nations Framework Convention on Climate Change meeting in Paris in 2016 the countries of the world agreed to a goal of limiting anthropogenic (human-caused) warming to 1.5° C above pre-industrial levels.[1] In order to achieve that goal, the Intergovernmental Panel on Climate Change (IPCC) has stated that we must decrease anthropogenic CO2 emissions (and therefore fossil fuel use) by 45% below 2010 levels by 2030, and that we must reach net-zero CO2 emissions by 2050.[2] If we wish to limit warming to 2.0° C above pre-industrial levels, we must decrease anthropogenic CO2 emissions by 25% below 2010 levels by 2030, and that we must reach net-zero CO2 emissions by 2070.
Media Attributions
- Figure 8.4.1 Steven Earle, CC BY 4.0
- Figure 8.4.2 Steven Earle, CC BY 4.0
- Figure 8.4.3 Steven Earle, CC BY 4.0
- Figure 8.4.4 Modified by Steven Earle, CC BY SA 4.0, based on AAPG data from Lovely and Ruggiero (1995, personal communication), via Wikimedia Commons, http://wiki.aapg.org/File:Sedimentar...is_fig4-55.png
- Figure 8.4.5 Steven Earle, CC BY 4.0
- Figure 8.4.6 Modified by Steven Earle, based on Hydraulic Fracturing diagram by Mike Norton, 2013, CC BY-SA 3.0, via Wikimedia, https://en.Wikipedia.org/wiki/Hydrau...HydroFrac2.svg)
- Every country in the world has signed the Paris Agreement, and so every person in the world needs to be on board with reaching its goals. Seven countries: Eritrea, Iran, Iraq, Libya, South Sudan, Turkey and Yemen have signed, but have not ratified the agreement. In 2019 the United States announced its withdrawal from the Paris Agreement, but that decision was reversed in February 2021. ↵
- IPCC. (2018). Summary for policymakers. In Masson-Delmotte, V., P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P.R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J.B.R. Matthews, Y. Chen, X. Zhou, M.I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, and T. Waterfield (Eds.), Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5° C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. World Meteorological Organization. https://www.ipcc.ch/site/assets/uplo..._report_LR.pdf ↵


