2.1: Minerals
- Page ID
- 25500
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Electrons, Protons, Neutrons and Atoms
Minerals are made up of atoms, and all atoms are made up of three main sub-atomic particles known as protons, neutrons and electrons. As summarized in Table 2.1.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. The negative charge of one electron balances the positive charge of one proton. Both protons and neutrons have a mass of 1, while electrons have almost no mass.
| Elementary particle | Charge | Mass |
| Electron | -1 | ~0 |
| Proton | 1 | 1 |
| Neutron | 0 | 1 |
The simplest atom is that of hydrogen (atomic number 1) which has one proton and one electron. The proton forms the nucleus, while the electron spins around it. All other elements have neutrons as well as protons in their nucleus, such as helium (atomic number 2). The positively-charged protons tend to repel each other, and the neutrons help to hold the nucleus together. The atomic number is the number of protons and the atomic weight is the number of protons plus neutrons. For hydrogen both the atomic number and weight are 1 because there is one proton and no neutrons. For helium the atomic number is 2 and the atomic weight is 4 because there are 2 protons and 2 neutrons. Figure 2.1.1 provides a simplified view of the elements up to number 36.

Electron orbits around the nucleus of an atom are arranged in what we call shells—also known as energy levels. The first shell can hold only two electrons, while the next shell will hold up to eight electrons. Subsequent shells can hold more electrons, but the outermost shell of any atom will hold no more than eight electrons. These outermost shells are important in bonding between atoms, and bonding takes place between atoms that do not have the full complement of eight electrons in their outer shells (or two in the first shell for the very light elements).
Bonding and Lattices
An atom seeks to have a full outer shell (i.e., 8 electrons for most elements, or 2 electrons for hydrogen, helium, lithium and beryllium[1] to be atomically stable. This is accomplished by lending, borrowing, or sharing electrons with other atoms. The noble gases, such as helium, neon, argon etc., already have their outer orbits filled so they don’t need to lose or gain electrons.
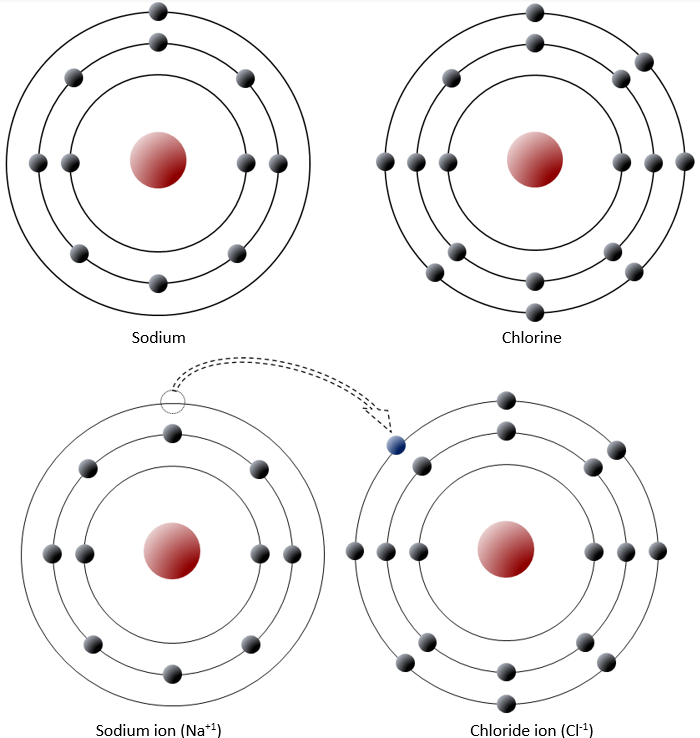
Sodium has 11 electrons, 2 in the first shell, 8 in the second, and 1 in the third (Figure \(\PageIndex{2}\)). Sodium readily gives up this third shell electron, and when it does it loses one negative charge and becomes positively charged. Chlorine, on the other hand, has 17 electrons, 2 in the first shell, 8 in the second, and 7 in the third. Chlorine readily accepts an eighth electron for its third shell, and therefore becomes negatively charged. In changing their number of electrons these atoms become ions—the sodium a positive ion or cation, the chlorine a negative ion or anion. The resulting electronic attraction between these ions is known as an ionic bond. Electrons can be thought of as being transferred from one atom to another in an ionic bond.
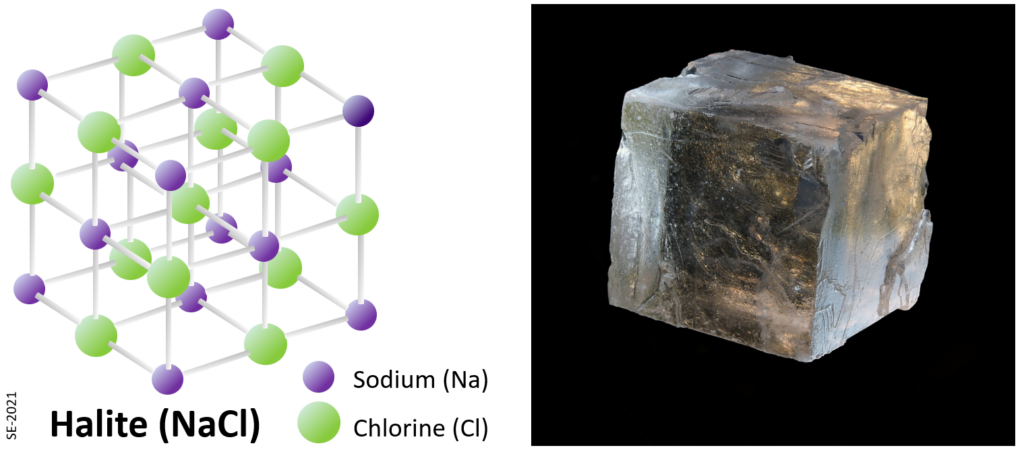
Common table salt (NaCl) is a mineral composed of chlorine and sodium linked together with ionic bonds (Figure \(\PageIndex{3}\)). The mineral name for NaCl is halite and its structure is cubic, meaning that all of the bonds are at right angles to each other. This why halite grows as cubic crystals (Figure \(\PageIndex{3}\), right), and it’s also why it breaks along three planes at right angles to each other (cleavage).
Exercise \(\PageIndex{1}\) Cations, Anions and Ionic Bonding
Several elements are listed below along with their atomic numbers. Assuming that the first electron shell can hold 2 electrons, and that subsequent electron shells can hold 8 electrons, sketch in the electron configurations for these elements. Predict whether the element is likely to form a cation (+) or an anion (-), and what valency it would have (e.g., +1, +2, -1 etc.) The first one is done for you.

Exercise answers are provided Appendix 2.
Element number 6 (carbon) has a 6 protons and 6 neutrons in its nucleus, and it also has 6 electrons—two in the first shell and 4 in the second. Carbon atoms would like to have either no electrons in the second shell, or 8, but both of those options are unlikely, so instead of losing or gaining electrons carbon atoms share electrons with other atoms, in many cases with other carbon atoms. This arrangement is depicted as a 2-dimensional structure on Figure \(\PageIndex{4}\), and the bonds between the inner carbon atom is known as a covalent bond. As this arrangement is extended out for millions of atoms (in either two or three dimensions) every carbon atom will be surrounded by 4 others, and each (except the very outermost ones) will have its need for 8 outer electrons fulfilled through sharing.

Covalent bonds are generally stronger than ionic bonds and carbon-carbon covalent bonds are the strongest of all, which is why diamond (which has nothing but covalent C-C bonds) is the hardest mineral and also why some man-made carbon compounds are extremely strong.
The elements silicon and oxygen are both abundant in the Earth’s crust and mantle and are present in the minerals that make up most rocks. Silicon and oxygen bond together to create a four-sided pyramid shape with an oxygen at each corner and a silicon in the middle, known as a silica tetrahedron (Figure \(\PageIndex{5}\)). This is the building block of the many important silicate minerals. The bonds in a silica tetrahedron have some of the properties of covalent bonds and some of the properties of ionic bonds. As a result of the ionic character, silicon becomes a cation (with a charge of +4) and oxygen becomes an anion (with a charge of -2). The net charge of a silica tetrahedron (Si04) is -4. As we will see later, silica tetrahedra are linked together in a variety of ways to form most of the common minerals of the crust.

Box 2.1 What’s With All of These “Sili” Names?
The element silicon is one of the most important geological elements and is the second-most abundant element in the Earth’s crust (after oxygen). Silicon bonds readily with oxygen to form a silica tetrahedron (Figure \(\PageIndex{5}\)). Pure silicon crystals are used to make semi-conductive media in electronic devices. A silicate mineral is one in which silicon and oxygen are present as silica tetrahedra (plural of tetrahedron). Silica also refers to a chemical component of a rock, and is expressed as % SiO2. The mineral quartz is made up entirely of silica tetrahedra, and some forms of quartz are known as silica. Silicone is a synthetic product (e.g., silicone rubber, resin or caulking) made from silicon-oxygen chains and various organic molecules. To help you keep the “sili” names straight, here is a summary table:
| Sili- Name | Details |
| Silicon | The 14th element |
| Silicon wafer | A crystal of pure silicon sliced very thinly and used for electronics |
| Silica tetrahedron | A combination of 1 silicon atom and 4 oxygen atoms that form a tetrahedron |
| % silica | The proportion of a rock that is comprised of the components Si and O2 |
| Silica | A form of the mineral quartz (SiO2) |
| Silicate mineral | A mineral that has silica tetrahedra in it (e.g., quartz, feldspar, mica, olivine, clay minerals, etc.) |
| Silicone | A flexible material (but not a mineral) made up of Si-O chains with attached organic molecules |
Non-Silicate Minerals and Mineral Groups
Elements bonded together in various configurations form minerals. A mineral is a naturally occurring, solid compound with a specific composition and a regular repeating lattice structure (like the halite in Figure 2.1.3). Most minerals are made up of a cation (a positively charged ion) or several cations, and an anion (a negatively charged ion) or an anion group. For example, in the mineral hematite (Fe2O3) the cation is Fe (iron – Fe3+) and the anion is O (oxygen O-2). (The two +3 iron ions contribute 6 positive charges and the three -2 oxygen ions contribute 6 negative charges, so the charge is balanced, which is a requirement of all minerals.) We group minerals into classes on the basis of their predominant anion or anion group. These include oxides, sulphides, carbonates and silicates, and others. Silicates are by far the predominant group in terms of their abundance within the crust and mantle, and they will be discussed later. Some examples of minerals from the different mineral groups are given in Table \(\PageIndex{2}\).
| Group | Examples |
| Oxides | hematite (iron-oxide – Fe2O3), corundum (aluminum-oxide Al2O3), water-ice (H2O) |
| Sulphides | galena (lead-sulphide – PbS), pyrite (iron-sulphide – FeS2), chalcopyrite (copper-iron-sulphide – CuFeS2) |
| Carbonates | calcite (calcium-carbonate – CaCO3), dolomite (calcium-magnesium-carbonate – (Ca,Mg)CO3 |
| Silicates | quartz (SiO2), feldspar (sodium-aluminum-silicate – NaAlSi3O8), olivine (iron or magnesium-silicate – (Mg,Fe)SiO4) (Note that in in quartz the anion is oxygen, and while it could be argued, therefore, that quartz is an oxide, it is always classed with the silicates.) |
| Halides | fluorite (calcium-flouride – CaF2), halite (sodium-chloride – NaCl) (Halide minerals have halogen elements as their anion – the minerals in the second last column on the right side of the Periodic Table, such as F, Cl, Br etc. (see Figure 2.1.1)) |
| Sulphates | gypsum (calcium-sulphate – CaSO4·H2O), barite (barium-sulphate – BaSO4) (Note that sulphates are different from sulphides. Sulphates have the SO4-2 ion while sulphides have the S-2 ion) |
| Native elements | gold (Au), diamond (C), graphite (C), sulphur (S) (see Figure 2.0.1), copper (Cu) |
Oxide minerals have oxygen as their anion, but they exclude those with oxygen complexes such as carbonate (CO3), sulphate (SO4), or silicate (SiO2). If the oxygen is also combined with hydrogen to form the hydroxyl anion (OH–) the minerals is known as a hydroxide. Some important hydroxides are limonite (FeO(OH)) and gibbsite (Al(OH)3), which are common within ores of iron and aluminum.
Sulphides are minerals with the S-2 anion, and they include galena (PbS), sphalerite (ZnS), chalcopyrite (CuFeS2) and molybdenite (MoS2), which are the most important ores of lead, zinc, copper and molybdenum respectively. Another important sulphide minerals is pyrite (FeS2).
Sulphates are minerals with the SO4-2 anion, and these include anhydrite (CaSO4 and its cousin gypsum CaSO4.2H20) and the sulphates of barium and strontium: barite (BaSO4) and celestite (SrSO4). In all of these cases the cation has a +2 charge which balances the -2 charge on the sulphate ion.
The halides are so named because the anions include the halogen elements chlorine, fluorine, bromine etc. Examples are halite (NaCl) and fluorite (CaF2).
The carbonates include minerals in which the anion is the CO3-2 complex. The carbonate combines with +2 cations to form minerals such as calcite (CaCO3), magnesite (MgCO3), dolomite ((Ca,Mg)CO3) and siderite (FeCO3). The copper minerals malachite and azurite are also carbonates.
In phosphate minerals the anion is PO4-4. An important phosphate mineral is apatite (Ca5(PO4)3(OH)), which is what your teeth are made of.
Native minerals include only one element (bonded to itself), such as gold, copper, sulphur or carbon (which could be graphite or diamond).
The silicate minerals include the elements silicon and oxygen in varying proportions ranging from SiO2 to SiO4. These are discussed at length below.
Exercise \(\PageIndex{2}\) Mineral Groups
We classify minerals according to the anion part of the mineral formula, and mineral formulas are always written with the anion part on the right. For example, for pyrite (FeS2) Fe2+ is the cation and S– is the anion. This helps us to know that it’s a sulphide, but it is not always that obvious. Hematite (Fe2O3) is an oxide, that’s easy, but anhydrite (CaSO4) is a sulphate because SO42- is the anion, not O. Along the same lines, calcite (CaCO3) is a carbonate, and olivine (Mg2SiO4) is a silicate. Minerals with only one element (such as S) are called native minerals, while those with an anion from the halogen column of the periodic table (Cl, F, Br, etc.) are halides. Provide group names for the following minerals:
| Name | Formula | Group | Name | Formula | Group |
| Sphalerite | ZnS | Sylvite | KCl | ||
| Magnetite | Fe3O4 | Silver | Ag | ||
| Pyroxene | MgSiO3 | Fluorite | CaF2 | ||
| Siderite | FeCO3 | Feldspar | KAlSi3O8 |
Exercise answers are provided Appendix 2.
Silicate Minerals
The vast majority of the minerals that make up the rocks of the earth’s crust are silicate minerals. These include minerals such as quartz, feldspar, mica, amphibole, pyroxene, olivine, and a great variety of clay minerals. The building block of all of these minerals is the silica tetrahedron, a combination of four oxygen atoms and one silicon atom. In silicate minerals these tetrahedra are arranged and linked together in a variety of ways, from single units to complex frameworks (Figure \(\PageIndex{7}\)). The simplest silicate structure, that of the mineral olivine, is composed of isolated tetrahedra bonded to iron and/or magnesium ions. In olivine the -4 charge of each silica tetrahedron is balanced by the addition of two divalent (i.e., +2) iron or magnesium cations. Olivine can be either Mg2SiO4 or Fe2SiO4, or some combination of the two, which is written like this: (Mg,Fe)2SiO4, meaning that any proportions of Mg and Fe are possible.

In pyroxene silica tetrahedra are linked together in a single chain, where one oxygen ion from each tetrahedron is shared with the adjacent tetrahedron, and hence there are fewer oxygens in the structure. The result is that the oxygen to silicon ratio is lower than in olivine (3:1 instead of 4:1), the net charge per silicon atom is atom is less (-2 instead of -4), and fewer cations are necessary to balance that charge. Pyroxene compositions are of the type MgSiO 3 , FeSiO 3 and CaSiO 3 , or some combination of these. In other words, pyroxene has one cation for each silica tetrahedron (e.g., MgSiO 3 ) while olivine has two (e.g., Mg 2 SiO 4 ).
In amphibole structures the silica tetrahedra are linked in a double chain that has an oxygen to silicon ratio lower than that of pyroxene, and hence still fewer cations are necessary to balance the charge. Amphibole is even more permissive than pyroxene and its compositions can be very complex. Hornblende, for example, can include sodium, potassium, calcium, magnesium, iron, aluminum, silicon, oxygen, fluorine and the hydroxyl ion (OH-1).
In mica structures the silica tetrahedra are arranged in continuous sheets. There is even more sharing of oxygens between adjacent tetrahedra and hence fewer charge-balancing cations are needed. Bonding between sheets is relatively weak, and this accounts for the well-developed one-directional cleavage (Figure \(\PageIndex{7}\)). Biotite mica can have iron and/or magnesium in it and that makes it a ferromagnesian silicate mineral (like olivine, pyroxene and amphibole). Chlorite is another similar mineral which commonly includes magnesium. In muscovite mica the only cations present are aluminum and potassium, and hence it is not a ferromagnesian silicate mineral.

Apart from muscovite, biotite and chlorite, there are many other sheet silicates (or phyllosilicates) which usually exist as clay-sized fragments (i.e., less than 0.004 mm). These include the clay minerals kaolinite, illite and smectite, and we will be looking at them more closely in Chapter 10.
Silica tetrahedra are bonded in three-dimensional frameworks in both the feldspars and in quartz. These minerals are non-ferromagnesian—they don’t contain any iron or magnesium. In addition to silica tetrahedra, the feldspars include aluminum, potassium, sodium and calcium in various combinations. The three main feldspar minerals are orthoclase (a.k.a., potassium feldspar), and two types of plagioclase: albite (sodium only) and anorthite (calcium only). Quartz contains only silica tetrahedra, so only has silicon and oxygen.
In quartz the silica tetrahedra are bonded in a “perfect” three-dimensional framework. Each tetrahedron is bonded to four other tetrahedra (with oxygen shared at each corner of each tetrahedron), and as a result, the ratio of silicon to oxygen is 1:2 (quartz is SiO2). Since the one silicon cation has a +4 charge and the two oxygen anions each have a -2 charge, the charge is balanced. There is no need for aluminum or any of the other cations such as sodium or potassium. The hardness, and lack of cleavage in quartz, are related to the fact that all of the bonds are the strong covalent/ionic bonds characteristic of the silica tetrahedron.
Box \(\PageIndex{2}\) Important Minerals
Before we move on to talk about rocks it may be useful to review some of the important things about minerals. First—and I cannot emphasize this enough—minerals and rocks are not the same. A mineral has a specific chemical composition and structure. Rocks are made up of minerals, just like words are made up of letters. You might be asked on a test to name a specific mineral or rock and it isn’t going to make anyone happy if you answer with a rock name for the mineral, or a mineral name for the rock. For most aspects of Environmental Geology you can get by if you are familiar with a relatively short list of minerals, as follows:
| Types of Minerals | Examples |
| Silicate minerals of common rocks | plagioclase feldspar, potassium feldspar, quartz, mica, amphibole, pyroxene and olivine |
| Clay minerals | kaolinite, illite, smectite, and serpentine (all are also silicates and are described in Chapter 10) |
| Others | hematite, magnetite (oxides), calcite and dolomite (carbonates), gypsum (sulphate), pyrite (sulphide) (plus a few other sulphide minerals that are discussed in Chapter 8) |
Media Attributions
- Figure 2.1.1 Image modified by Steven Earle, from a CC0 1.0, image Periodic Table by Laszlo Nemeth, via Wikimedia Commons, https://commons.wikimedia.org/wiki/F..._simple_eo.svg
- Figure 2.1.2 Steven Earle, CC BY 4.0
- Figure 2.1.3 Left: Steven Earle, CC BY 4.0; Right: Cubed Shaped Crystal by Hans-Joachim Engelhardt, CC-BY-SA-4.0, via Wikimedia Commons, https://commons.wikimedia.org/wiki/F..._KUBUS_50P.jpg
- Figure 2.1.4 Steven Earle, CC BY 4.0
- Figure 2.1.5 Steven Earle, CC BY 4.0
- Figure 2.1.6 Steven Earle, CC BY 4.0
- Figure 2.1.7 Steven Earle, CC BY 4.0
- Figure 2.1.8 Steven Earle, CC BY 4.0
- In fact, for beryllium losing one electron means losing its entire outer shell. ↵


