3.4: Minerals
- Page ID
- 20761
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Atoms and Isotopes
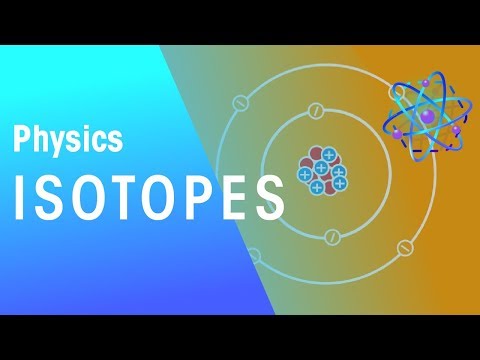
A chemical element is a substance that cannot be made into a more straightforward form by ordinary chemical means the smallest unit of a chemical element is an atom. An atom has all the properties of that element. At the center of an atom is a nucleus made up of subatomic particles called protons and neutrons. Protons have a positive electrical charge. The number of protons in the nucleus determines what element the atom is. Neutrons are about the size of protons but have no charge. Electrons, each having a negative electrical charge, orbit the nucleus at varying energy levels in a region known as the electron cloud.
Because electrons are minuscule compared with protons and neutrons, the number of protons plus neutrons gives the atom its atomic mass. All atoms of a given element always have the same number of protons but may differ in the number of neutrons found in its nucleus. Atoms of an element with differing numbers of neutrons are called isotopes. (Matter Matters | Earth Science, n.d.)
Ions and Molecules
Atoms are stable when they have a full outermost electron valence shell. For an atom to fill its outermost shell, it will give, take, or share electrons. When an atom either gains or loses electrons, this creates an ion. Ions have either a positive or a negative electrical charge. What is the charge of an ion if the atom loses an electron? An atom with the same number of protons and electrons has no overall charge, so if an atom loses the negatively charged electron, it has a positive charge. What is the charge of an ion if the atom gains an electron? If the atom gains an electron, it has a negative charge.
When atoms chemically bond, they form compounds. The smallest unit of a compound with all the properties of that compound is a molecule. When two or more atoms share electrons to form a chemical bond, they form a molecule. The molecular mass is the sum of the masses of all the atoms in the molecule.
Chemical Bonding
Ions come together to create a molecule so that electrical charges are balanced; the positive charges balance the negative charges, and the molecule has no electrical charge. An atom will balance its electrical charge by sharing an electron with another atom, giving it away, or receive an electron from another atom.
The joining of ions to make molecules is chemical bonding. There are three main types of chemical bonds. Ionicbonds occur when electrons are transferred between atoms. Covalentbonds occur when an atom shares one or more electrons with another atom. The sharing of electrons is not always evenly distributed within a molecule. If one atom has the electrons more often than another atom in the molecule, it has a positive and a negative side. It is a polar molecule because it acts a little bit as if it had poles, like a magnet. Finally, hydrogen bonds are weak intermolecular bonds that form when the positive side of one polar molecule is attracted to another polar molecule’s negative side.
Minerals
Minerals are categorized based on their chemical composition. Owing to similarities in composition, minerals within the same group may have similar characteristics. Geologists have a precise definition of minerals. A material is characterized as a mineral if it meets all the following traits:
- Inorganic, crystalline solid.
- Formed through natural processes and has a definite chemical composition.
- Identified by their characteristic physical properties such as crystalline structure, hardness, density, flammability, and color.
Crystalline Solid
Minerals are crystalline solids. A crystal is a solid in which the atoms are arranged in a regular, repeating pattern. The pattern of atoms in different samples of the same mineral is the same. Is glass a mineral? Without a crystalline structure, even natural glass is not a mineral. (Minerals and Mineral Groups | Earth Science, n.d.)
Organic and Inorganic Substances
Organic substances are the carbon-based compounds made by living creatures and include proteins, carbohydrates, and oils. Inorganic substances have a structure that is not characteristic of living bodies. Coal is made of plant and animal remains. Is it a mineral? Coal is classified as a sedimentary rock but is not a mineral. (Minerals and Mineral Groups | Earth Science, n.d.)
Natural Processes
Minerals are made by natural processes, those that occur in or on Earth. A diamond created deep in Earth’s crust is a mineral. Is a diamond created in a laboratory by placing carbon under high pressures a mineral? No. Do not buy a laboratory-made “diamond” for jewelry without realizing it is not technically a mineral.
Chemical Composition
Nearly all (98.5 percent) of Earth’s crust is made up of only eight elements – oxygen, silicon, aluminum, iron, calcium, sodium, potassium, and magnesium – and these are the elements that make up most minerals.
All minerals have a specific chemical composition. The mineral silver is made up of only silver atoms and diamond is made only of carbon atoms, but most minerals are made up of chemical compounds. Each mineral has its chemical formula. Halite is NaCl (sodium chloride). Quartz is always made of two oxygen atoms bonded to a silicon atom, SiO2. If a mineral contains any other elements in its crystal structure, it is not quartz.
A hard mineral with covalently bonded carbon is diamond, but a softer mineral containing calcium and oxygen along with carbon is calcite. Some minerals have a range of chemical composition. Olivine always has silicon and oxygen, as well as iron or magnesium or both. (Minerals and Mineral Groups | Earth Science, n.d.)
Mineral Identification
Their physical characteristics can identify minerals. The physical properties of minerals are related to their chemical composition and bonding. Some characteristics, such as a mineral’s hardness, are more useful for mineral identification. Color is readily observable and undoubtedly visible, but it is usually less reliable than other physical properties. (Reading: Physical Characteristics of Minerals | Geology, n.d.)
Color
Diamonds are popular gemstones because the way they reflect light makes them very sparkly. Turquoise is prized for its striking greenish-blue color. Notice that specific terms are being used to describe the appearance of minerals. Color is rarely useful for identifying a mineral.
Streak
Streak is the color of a mineral’s powder. Streak is a more reliable property than color because streak does not vary. Minerals that are the same color may have a different colored streak. Many minerals, such as the quartz, do not have a streak. To check streak, scrape the mineral across an unglazed porcelain plate. Yellow-gold pyrite has a blackish streak, another indicator that pyrite is not gold, with a golden yellow streak.
Luster
Luster describes the reflection of light off a mineral’s surface. Mineralogists have specific terms to describe luster. One straightforward way to classify luster is based on whether the mineral is metallic or non-metallic. Minerals that are opaque and shiny, such as pyrite, have a metallic luster. Minerals such as quartz have a non-metallic luster.
Specific Gravity
Density describes how much matter is in a certain amount of space: density = mass/volume. Mass is a measure of the amount of matter in an object. Its volume describes the amount of space an object takes up. The density of an object depends on its mass and its volume. For example, the water in a drinking glass has the same density as the water in the same volume of a swimming pool. Gold has a density of about 19 g/cm3; pyrite has a density of about 5 g/cm3 – that is another way to tell pyrite from gold. Quartz is even less dense than pyrite and has a density of 2.7 g/cm3. The specific gravity of a substance compares its density to that of water. Denser substances have higher specific gravity.
Hardness
Hardness is a measure of whether a mineral will scratch or be scratched. Mohs Hardness Scale is a reference for mineral hardness. With a Mohs scale, anyone can evaluate an unknown mineral for its hardness. Imagine you have an unknown mineral. You find that it can scratch fluorite or even apatite, but feldspar scratches it. This examination of the mineral informs you that the mineral’s hardness is between 5 and 6. Note that no other mineral can scratch diamond. Breaking a mineral breaks its chemical bonds. Since some bonds are weaker than other bonds, each type of mineral is likely to break where the bonds between the atoms are weakest. For that reason, minerals break apart in distinctive ways. (Mineral Identification | Earth Science, n.d.)
Cleavage
Cleavage is the tendency of a mineral to break along specific planes to make smooth surfaces. Halite breaks between layers of sodium and chlorine to form cubes with smooth surfaces. One reason gemstone is beautiful is that the cleavage planes make an attractive crystal shape with smooth faces. (Mineral Identification | Earth Science, n.d.)
Fracture
Fracture is a break in a mineral that is not along a cleavage plane. Fracture is not always the same in the same mineral because its structure does not determine fracture. Minerals may have natural fractures. Metals usually fracture into jagged edges. If a mineral splinter like wood, it may be fibrous. Some minerals, such as quartz, form smooth curved surfaces when they fracture. (Reading: Physical Characteristics of Minerals | Geology, n.d.)
Other Identifying Characteristics
Mineral Formation
Minerals form under an enormous range of geologic conditions. There are more ways to form minerals than there are types of minerals themselves. Minerals can form from volcanic gases, sediment formation, oxidation, crystallization from magma, or deposition from a saline fluid. (Reading: Physical Characteristics of Minerals | Geology, n.d.)
Formation from Hot Material
A rock is a collection of minerals. Imagine a rock that becomes so hot it melts. Many minerals start in liquids that are hot enough to melt rocks. Magma is melted rock inside Earth, a molten mixture of substances that can be hotter than 1,000oC. Magma cools slowly inside Earth, which gives mineral crystals time to grow large enough to be seen clearly. When magma erupts onto Earth’s surface, it is called lava. Lava cools much more rapidly than magma when it is below the surface. In cooling lava, mineral crystals do not have time to form and are exceedingly small. The chemical composition will be the same as if the magma cooled slowly. Existing rocks may be heated enough to release the molecules from their structure and move around. The molecules may match up with different molecules to form new minerals as the rock cools, a process called metamorphism. (Matter Matters | Earth Science, n.d.)
Formation from Solutions
Water on Earth, such as the water in the oceans, contains chemical elements mixed into a solution. Various processes can cause these elements to combine to form solid mineral deposits.
Minerals from Salt Water
When water evaporates, it leaves behind a solid precipitate of minerals. Water can only hold a certain amount of dissolved minerals and salts. When the amount is too vast to stay dissolved in the water, the particles come together to form mineral solids, which sink. Halite readily precipitates out of the water, as does calcite. Some lakes, such as Mono Lake in California or The Great Salt Lake in Utah, contain many mineral precipitates.
Minerals from Hot Underground Water
Magma heats nearby underground water, which reacts with the rocks around it to pick up dissolved particles. As the water flows through open spaces in the rock and cools, it deposits solid minerals. The mineral deposits form when a mineral fills cracks in rocks are called veins when minerals are deposited in open spaces and large crystal forms.
Mining and Mineral Use
Some minerals are beneficial. An ore is a rock that contains minerals with useful elements. Aluminum in bauxite ore is extracted from the ground and refined to be used in aluminum foil and many other products. The cost of creating a product from a mineral depends on how abundant the mineral is and how much the extraction and refining processes cost. Environmental damage from these processes is often not figured into a product’s cost. It is crucial to use mineral resources wisely.
Finding and Mining Minerals
Geologic processes create and concentrate minerals that are valuable natural resources. Geologists study geological formations and test the physical and chemical properties of soil and rocks to locate possible ores and determine their size and concentration. A mineral deposit will only be mined if it is profitable. A concentration of minerals is only called an ore deposit if it is profitable to mine. There are many ways to mine ores. (Reading: Mining and Mineral Use | Geology, n.d.)
Surface mining allows the extraction of ores that are close to Earth’s surface. Overlying rock is blasted, and the rock that contains the valuable minerals is placed in a truck and taken to a refinery. Surface mining includes open-pit mining and mountaintop removal. Other methods of surface mining include strip mining, placer mining, and dredging. Strip mining is like open-pit mining but with material removed along a strip.
Placers are valuable minerals found in stream gravels. California’s nickname, the Golden State, can be traced to the discovery of placer deposits of gold in 1848. The gold weathered out of hard metamorphic rock in the western Sierra Nevada, which also contains deposits of copper, lead, zinc, silver, chromite, and other valuable minerals. The gold traveled down rivers and then settled in gravel deposits. Currently, California has active mines for gold and silver and for non-metal minerals such as sand and gravel, which are used for construction. (Reading: Mining and Mineral Use | Geology, n.d.)

Underground mining is used to recover ores that are deeper into Earth’s surface. Miners blast and tunnel into the rock to gain access to the ores. How underground mining is approached depends on the placement of the ore body, its depth, concentration of ore, and the surrounding rock’s strength. Underground mining is costly and dangerous. Fresh air and lights must also be brought into the tunnels for the miners, and accidents are far too frequent.
The ore’s journey to becoming a useable material is only just beginning when the ore leaves the mine. Rocks are crushed so that the valuable minerals can be separated from the waste rock. Then the minerals are separated from the ore. A few methods for extracting ore are:
- Heap leaching: the addition of chemicals, such as cyanide or acid, to remove ore.
- Flotation: the addition of a compound that attaches to the valuable mineral and floats.
- Smelting: roasting rock, causing it to segregate into layers so the mineral can be extracted.
To extract the metal from the ore, the rock is melted at a temperature higher than 900 degrees Celsius, which requires much energy. Extracting a metal from the rock is so energy-intensive that recycling just 40 aluminum cans will save the energy equivalent of one gallon of gasoline. (Mining and Mineral Use | Earth Science, n.d.)
Although mining provides people with many necessary resources, environmental costs can be high. Surface mining clears the landscape of trees and soil, and nearby streams and lakes are inundated with sediment. Pollutants from the mined rock, such as heavy metals, enter the sediment and water system. Acids flow from some mine sites, changing the composition of nearby waterways.
U.S. law has changed so that in recent decades a mine region must be restored to its natural state, a process called reclamation. This is not true of older mines. Pits may be refilled or reshaped, and vegetation planted. Pits may be allowed to fill with water and become lakes or maybe turned into landfills. Underground mines may be sealed off or left open as homes for bats.
Some minerals are valuable because they are beautiful. Jade has been used for thousands of years in China. Diamonds sparkle on many engagement rings. Minerals like jade, turquoise, diamonds, and emeralds are gemstones. A gemstone, or gem, is a material that is cut and polished for jewelry. Gemstones are usually rare and do not break or scratch easily. Most are cut along cleavage faces and then polished so that light bounces back off the cleavage planes. Light does not pass through opaque gemstones, such as turquoise. Gemstones are not just used in jewelry. Diamonds are used to cut and polish other materials, such as glass and metals because they are so hard. The mineral corundum, of which ruby and sapphire are varieties, is used in sandpaper products.
Minerals are used in much less obvious places. The mineral gypsum is used for the sheetrock in homes. Window glass is made from sand, which is mostly quartz. Halite is mined for rock salt. Copper is used in electrical wiring, and bauxite is the source of the aluminum used in soda cans. (Reading: Mining and Mineral Use | Geology, n.d.)
Conflict Resouces
Blood Diamonds
Blood diamonds, also called conflict diamonds, are diamonds mined in conflict areas and sold to finance warlords or invading armies in a geographic region. The term “blood” diamonds are used to highlight the negative impacts and consequences of diamonds mined for these ends. Often, women and children are enslaved to mine blood diamonds, and other conflict resources, to fund these local and regional wars. The Kimberley Process was created to help combat and regulate the flow of diamonds mined around the world to ensure that people were not purchasing blood diamonds. Blood diamonds have been mined from Angola, Ivory Coast, Sierra Leone, Liberia, Guinea, and Guinea Bissau.
Cobalt
A new form of conflict mineral has developed in recent years. With the advancement of technology (i.e. laptops, smartphones, tablets), the need and demand for the mineral cobalt have grown rapidly. It is believed that Subsaharan Africa, specifically Central Africa, has roughly two-thirds of the world’s cobalt. To meet the demand, women and children have been imprisoned to extract cobalt from the mines. Currently, there is not a formalized process to track and monitor where cobalt comes from. Technology companies like Apple, Samsung, Dell, and others are looking at creating their own supply chain for cobalt so that where the cobalt comes from can be monitored.