4.12: Soil Nutrient Cycling
- Last updated
- Save as PDF
- Page ID
- 22346
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Joann Whalen, Noura Ziadi, Jeff Schoenau, Maxime C. Paré, David Burton, and Tom Bruulsema
Learning Objectives
On completion of this chapter, students will be able to:
- Know the forms of soil nutrients that can be acquired by plants
- Know the factors controlling nutrient transformations into plant-available forms
- Understand how to evaluate soil fertility with soil testing and plant analysis
- Understand that inorganic and organic fertilizers can be applied to replenish the soil nutrient supply
- Be familiar with the 4R Nutrient Stewardship approach to soil nutrient management
- Be aware of the environmental implications of soil nutrient management
- Be acquainted with some examples of soil fertility improvement in Canada
INTRODUCTION
Soils support plant growth. Seeds germinate in the warm, moist soil environment. The seedling emerges at the soil surface, which needs to be porous and uncompacted, with no surface crust. Elongation of the radical and development of the root system occurs easily in soil with a crumbly, loose structure. The root system has two vital functions. The first is anchorage. Many plants grow a thick, strong primary root that is approximately as tall as the stem. A plant that is firmly rooted in the soil is unlikely to be dislodged by normal rainfall events and windstorms. The second vital function is resource acquisition, since most of the water and many essential elements for plant growth are absorbed through the root hairs (Table 7.1).
Table 7.1. Essential plant elements, and the forms of nutritive cations, anions and molecules absorbed by plant roots (adapted from Havlin et al., 2014)
| Essential element | Concentration | Nutritive form(s) absorbed | ||
|---|---|---|---|---|
| (μg g-1 dry mass) | Cation | Anion | Molecule | |
| C | 450000 | CO2 1 | ||
| H | 450000 | H2O 2 | ||
| O | 60000 | CO2, H2O | ||
| N | 15000 | NH4+ | NO3- | N2 3 |
| K | 10000 | K+ | ||
| Ca | 5000 | Ca2+ | ||
| P | 2000 | HPO42-, H2PO4- | ||
| S | 2000 | SO42- | SO2 4 | |
| Mg | 1000 | Mg2+ | ||
| Fe | 100 | Fe2+, Fe3+ | Soluble chelate 5 | |
| Cl | 100 | Cl- | ||
| Mn | 50 | Mn2+ | Soluble chelate | |
| B | 20 | H2BO3- | H3BO3, soluble chelate | |
| Zn | 20 | Zn2+ | Soluble chelate | |
| Cu | 6 | Cu2+ | Soluble chelate | |
| Mo | 0.1 | MoO4- | Soluble chelate | |
| Ni | 0.01 | Ni2+ | Soluble chelate | |
| 1 CO2 gas is absorbed through leaves and converted to carbohydrates (CH2O)n via photosynthesis. 2 H2O, as water vapour or liquid water, is absorbed through foliage and roots. 3 Symbiotic and associative N2-fixing prokaryotes that grow with the plant transform N2 gas into NH4+ via biological nitrogen fixation, and then transported into plant cells. 4 Less than 10% of the plant’s sulphur requirement comes from SO2 gas absorbed through leaves. 5 Soluble chelates are readily absorbed by the root. |
||||
Soil fertility refers to the ability of soil to supply nutrient ions for plant growth. Soils have inherent fertility, which depends upon four factors:
- parent material, which is weathered to release nutrients;
- vegetation, which produces organic residues that are decomposed to release nutrients;
- organisms that participate in weathering and are responsible for decomposition and nutrient cycling; and
- climate, which determines the rates of biological, chemical and physical processes that contribute to or deplete the soil nutrient supply.
Inherent soil fertility is a good predictor of what plant species will grow in a given area under natural conditions, without human intervention. When we harvest agricultural crops to feed people and livestock, we remove some nutrients from the soil-plant system. Consequently, agroecosystems are usually fertilized to replenish or increase the soil nutrient supply. Selecting the right fertilizer and other soil amendments requires careful decision, since it is expensive to purchase and apply these materials to agricultural land, and because overuse can have negative environmental consequences. Farms across Canada can follow nutrient management plans to guide the application of fertilizing materials to agricultural soils, for the best agronomic, economic and environmental outcomes.
NUTRIENTS IN THE SOIL-PLANT SYSTEM
Fine roots and root hairs grow in soil pores, which contain air and water. Soil pore water is a dilute electrolyte solution containing water-soluble nutrient ions. To satisfy their water requirements, plants absorb water from soil via osmosis and rely on aquaporins to selectively conduct water molecules in and out of cells, which regulates the cell volume and internal osmotic pressure. Water-soluble ions in the soil pore water enter the root hairs and diffuse through the epidermis. However, the root surface must maintain electrical neutrality. Any ion that enters the root causes the release of a counter-ion such as H+, OH– or HCO3– (Figure 7.1). For example, when a positively charged ion such as potassium (K+, a cation) passes through the root epidermis, the root releases one H+ ion into the surrounding soil pore water, and absorption of a calcium ion (Ca2+) results in the release of 2 H+ into the soil pore water. Anion absorption is similarly balanced, such that uptake of nitrate (NO3–) causes the root to release an OH– ion or a HCO3– ion into the soil pore water.
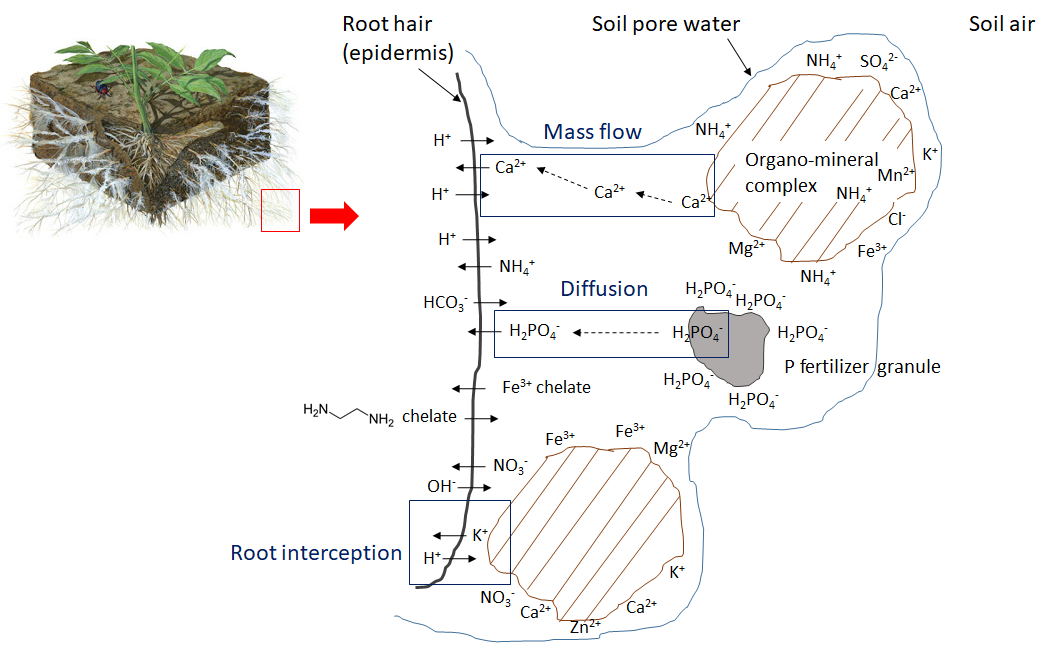
Once inside the root, water-soluble ions can move through the apoplast of the cortex, a transport pathway that transports water and dissolved substances along cell walls and extracellular space outside of plasma membranes. There are two other pathways for water-soluble ion movement: the symplastic and transmembrane pathways. In the symplastic pathway, water and solutes move along the cytosol and cross the plasma membranes of adjacent cells via the plasmodesmata. Alternatively, in the transmembrane route, the dissolved minerals and water move from cell to cell by crossing the cell wall, exiting one cell before they enter the next cell. The suberized layer surrounding the endodermis acts as a physical barrier, and ions must be transported through the plasma membrane via protein channels by binding with transporter proteins. Some of the transporter proteins responsible for nutrient ion flux in plants are listed in Table 7.2. Next, nutrient ions enters the xylem and are transported along with water to the stem, leaves, flowers, seeds and other components. Nutrient ions may be incorporated into structural compounds within these plant organs, or support metabolic processes in the cytoplasm. Furthermore, nutrient ions may be remobilized and translocated to other tissues or organs if they are needed in another part of the plant.
Table 7.2. Transporter proteins mediate the flux of nutrient ions across the plasma membranes of vascular plants. Adapted from Reid and Hayes (2003)
| Nutrient ion | Transporter protein | Comments |
|---|---|---|
| NH4+ | High affinity AMT transporter proteins | |
| NO3- | Nitrate transporter protein (NRT2) family | |
| H2PO4- and HPO42- |
PHT1 transporter protein | PHT1;1 through PHT1;9 were identified in Arabidopsis thaliana |
| K+ | K+ transporter protein (Trk) family | |
| SO42- | SO42- transporter proteins | Plants also possess the cysteine (Cys) transporter, methionine (Met) transporter, and glucosinolate (GSL) transporter |
| Ca2+ | Ca2+ transporters, Ca2+-ATPases and H+/Ca2+ antiporters | Transporter proteins that move Ca2+ across the membrane, against the electrochemical gradient, are specifically referred to as an ion pump. |
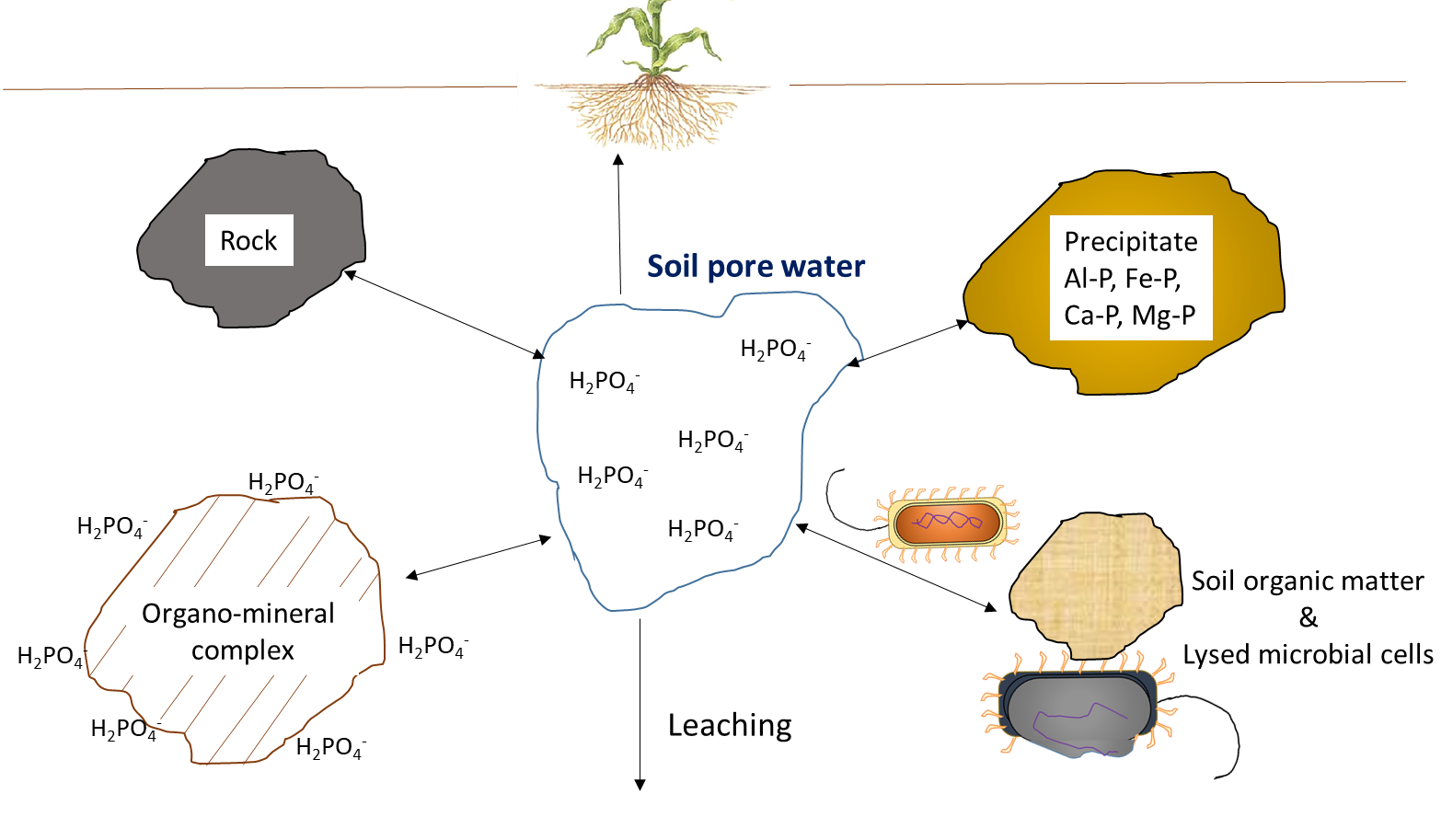
Plant uptake depletes the nutrient ion concentration in soil. Ions may move together with water through mass flow or be intercepted by growing roots that make contact with exchangeable ions on organo-mineral surfaces, a process referred to as root interception (Figure 7.1). Ions may also move across diffusion gradients from a zone of high concentration to a zone of low concentration in pore water close to the root (Figure 7.1). The concentration of nutrient ions in soil pore water is also affected by multiple, concurrent biological, chemical and physical processes, illustrated in Figure 7.2.
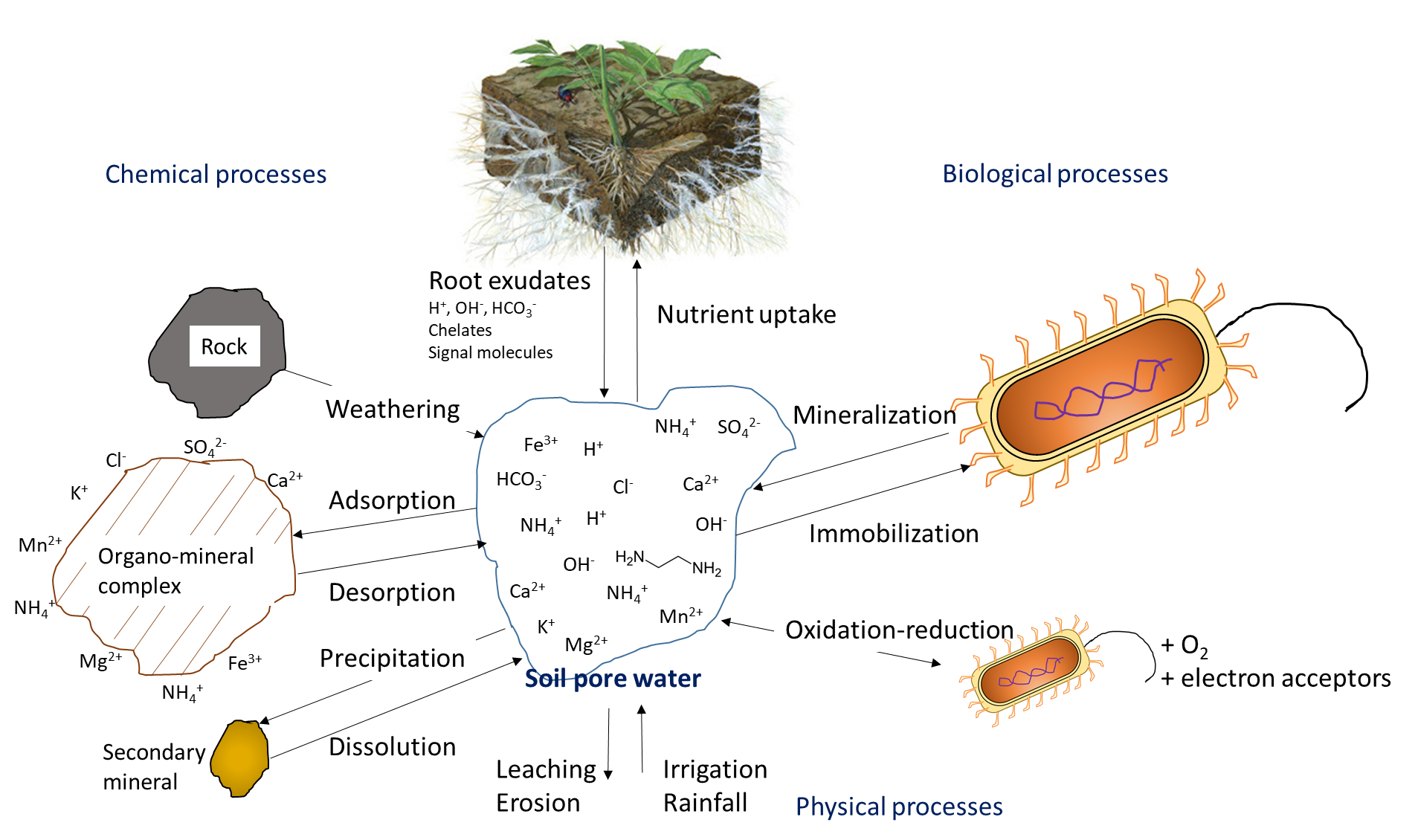
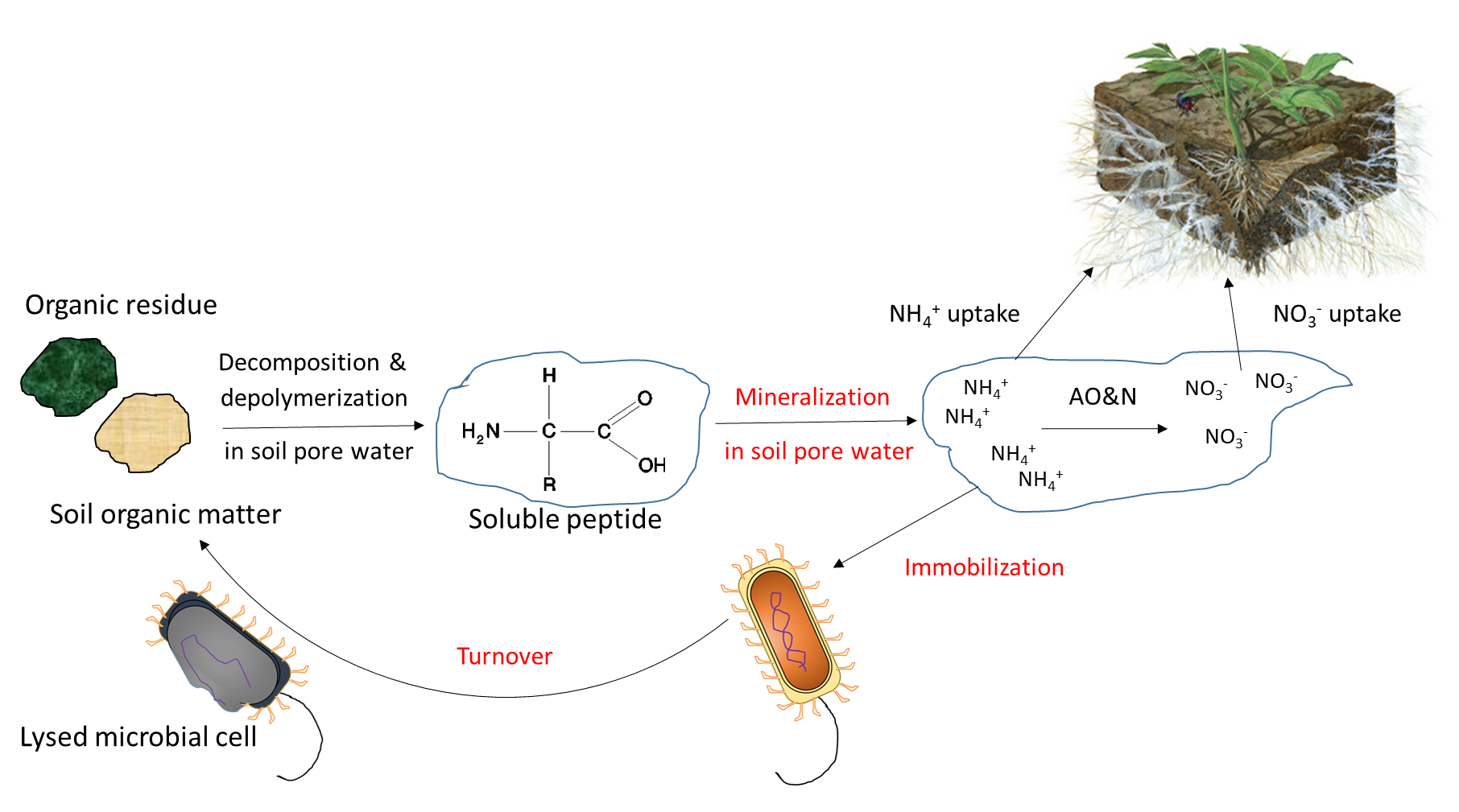
Biological activity is responsible for the mineralization of organic compounds, which releases water-soluble nutrient ions. Mineralization requires a specific hydrolytic enzyme, produced by plant roots or soil microorganisms, to cleave the covalent bonds in an organic compound. Soil enzymes function in the presence of water, often outside of biological cells. Mineralization of soil organic matter and organic residues releases ammonium (NH4+), phosphate (HPO42-, H2PO4–) and sulfate (SO42-) into the soil pore water. Immobilization refers to absorption of nutrient ions by organisms other than plants. Ions are immobilized in soil microbial biomass for a limited period of time (days to weeks) while the microorganism grows and reproduces, and are released at the end of their lifespan. The mineralization-immobilization-turnover concept applies to any nutrient that is covalently bound in organic matter, including carbon, nitrogen, phosphorus and sulphur. An illustration of the concept (Figure 7.3) shows how microorganisms produce enzymes to hydrolyze proteins, releasing NH4+ in the soil pore water, which is then immobilized inside growing microbial cells, and finally re-released into the soil pore water upon lysis of those cells.
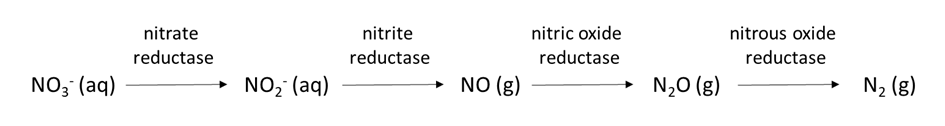
The oxygen concentration in soil pore water affects the transformation of nutrient ions. Oxygen moves approximately 1000 times faster through air-filled pores than through soil water, so it is not uncommon for oxygen limitation to occur in soil pore water. Limited oxygen availability can stimulate denitrification, a biological reaction in which the nitrogen electron acceptors gain electrons and decrease their oxidation state, e.g.,
NO3–(aq) → NO2–(aq) → NOx(g) → N2O (g) → N2(g)
which also changes the chemical form of nitrogen from water-soluble NO3– to dinitrogen (N2) gas. Chemical reduction of elements such as Mn, Fe, and sulfate in anoxic (low-oxygen) soil is another process that alters the chemical speciation and quantities of these nutrient ions in soil pore water.
Chemical reactions that add nutrient ions to soil pore water include weathering, which occurs when the broken, exposed surfaces of mineral surfaces are exposed to acidic compounds, such as carbonic acid. Dissolution refers to the solubilisation of nutrient ions from clays and colloidal materials in the presence of water. In dry soils, dissolution is less common because a low volume of soil pore water will increase the proximity of nutrient ions with reactive ions and molecules that can form precipitates. Surface complexation reactions on organo-mineral surfaces can either increase the concentration of nutrient ions, through desorption reactions, or remove ions from the soil solution through adsorption reactions.
The concentration of nutrient ions is highly dependent on the water content in soil pores. Irrigation and rainfall add water to the soil pores, thereby diluting the water-soluble nutrient ions. In humid regions that have more precipitation than evapotranspiration, soil pores may periodically reach 100% water-filled pore space. This will limit oxygen diffusion, which affects oxidation-reduction reactions, and impedes the growth of most plants, except those with morphological adaptations that allow them to tolerate inundation. Agricultural activities generally occur on land that has good natural drainage or where drainage systems are installed to encourage water movement below the cultivable topsoil. Drainage removes water, but it also leaches water-soluble nutrient ions through the profile, along with the water. Removal of excess water from topsoil via drainage or through evaporation will increase the relative concentration of water-soluble ions remaining in soil pore water.
SOIL FERTILITY AND PLANT NUTRITION
Plants acquire most of their essential nutrients ions from the soil pore water, a dynamic environment. In agriculture, much effort is made to understand the soil nutrient supply. Does a particular soil have enough nutrients to support the plant needs, or do we need to add fertilizer containing nutrients to avoid a plant nutrient deficiency?
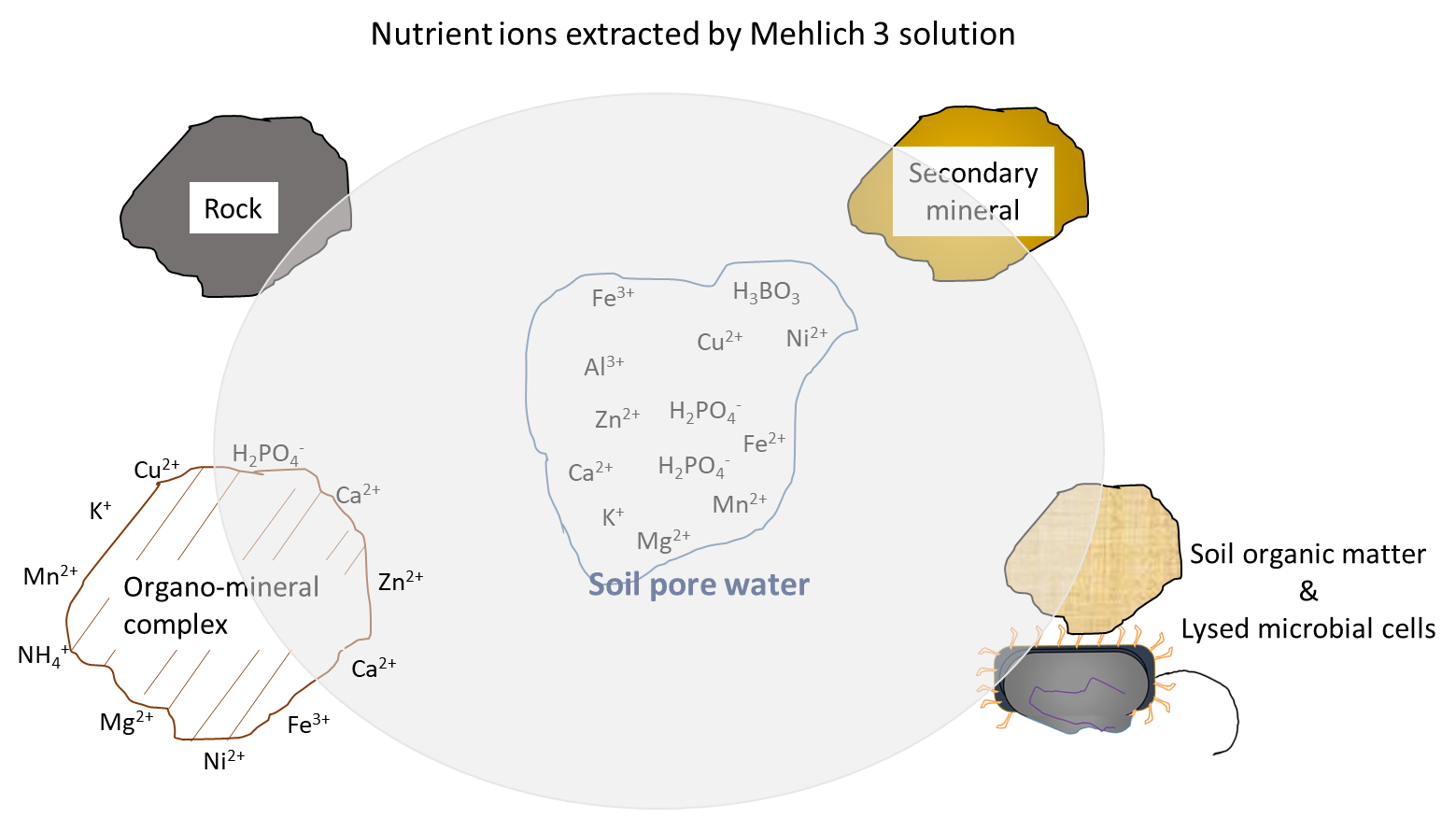
Soil fertility testing is an accurate way to assess the nutrient supply in agricultural soil. The main purpose of soil fertility testing is to determine whether a soil contains sufficient nutrients to meet the needs of an annual crop during its lifespan (one growing season). In addition, soil fertility testing can determine whether there is an adequate nutrient supply for perennial crops during their establishment and production phases, which span multiple growing seasons. Soil fertility testing relies on chemical and biological analyses by certified laboratories. The results of these tests are a good indicator of the plant-available nutrient supply. This concept relies on an understanding of two facts. First, the plant will acquire nutrient ions from the soil pore water throughout the growing season according to its nutrient demands. Second, the soil pore water will be replenished with water-soluble nutrient ions at a rate that reflects the combined biological, chemical and physical processes that contribute and remove ions from the soil pore water, during the growing season. For this reason, soil testing methods such as the Mehlich 3 extraction remove nutrient ions from soil pore water and other sources (Figure 7.4), a good approximation of the nutrient supply that will become available to plants during the growing season.
Another purpose of soil fertility testing is to assess the probability of a profitable response to fertilizers and soil amendments. This evaluation is part of an integrated plan to improve the growing conditions for crops. Farms set yield targets for their crops, according to the production goals and economic returns they hope to achieve. The maximum yield that can be expected for a particular crop depends on its genetic and physiological characteristics, which govern the plant’s photosynthetic capacity, its ability to acquire water and nutrients from soil, and its ability to convert these resources into grain, oilseed, starchy tubers, fruit or other marketable products. Furthermore, the maximum yield is influenced by the plant’s response to environmental conditions during the growing season. Unfavorable weather can provide too much or too little rainfall for the crop. Heat stress resulting from too hot temperatures during summer months, or frost damage in the spring and fall months, are stressful for crops. Farms also follow good agronomic practices to control growth limiting factors. For instance, the number of plants occupying a given area is optimized to intercept all available solar radiation, but too-close spacing could cause competition among plants and should be avoided. Weeds, pests and disease-causing organisms must be controlled to prevent yield losses. Finally, soils need to provide adequate nutrients, at the right time during crop development, to achieve the expected yield and quality outcomes.
Can You Dig It!
When Blueberry Gets Wild with Nitrogen Fertilizer
The availability of nitrogen (N) in the soil solution limits plant growth and crop yields in most agroecosystems. That is why farmers often fertilize their fields with one or several forms of N. Nevertheless, the efficacy of N fertilization depends on several factors including the presence of weeds, which can compete with the crop for the acquisition of fertilizer-derived N. Inappropriate N fertilization can indeed be counterproductive as it can stimulate weed growth rather than that of the targeted crop.
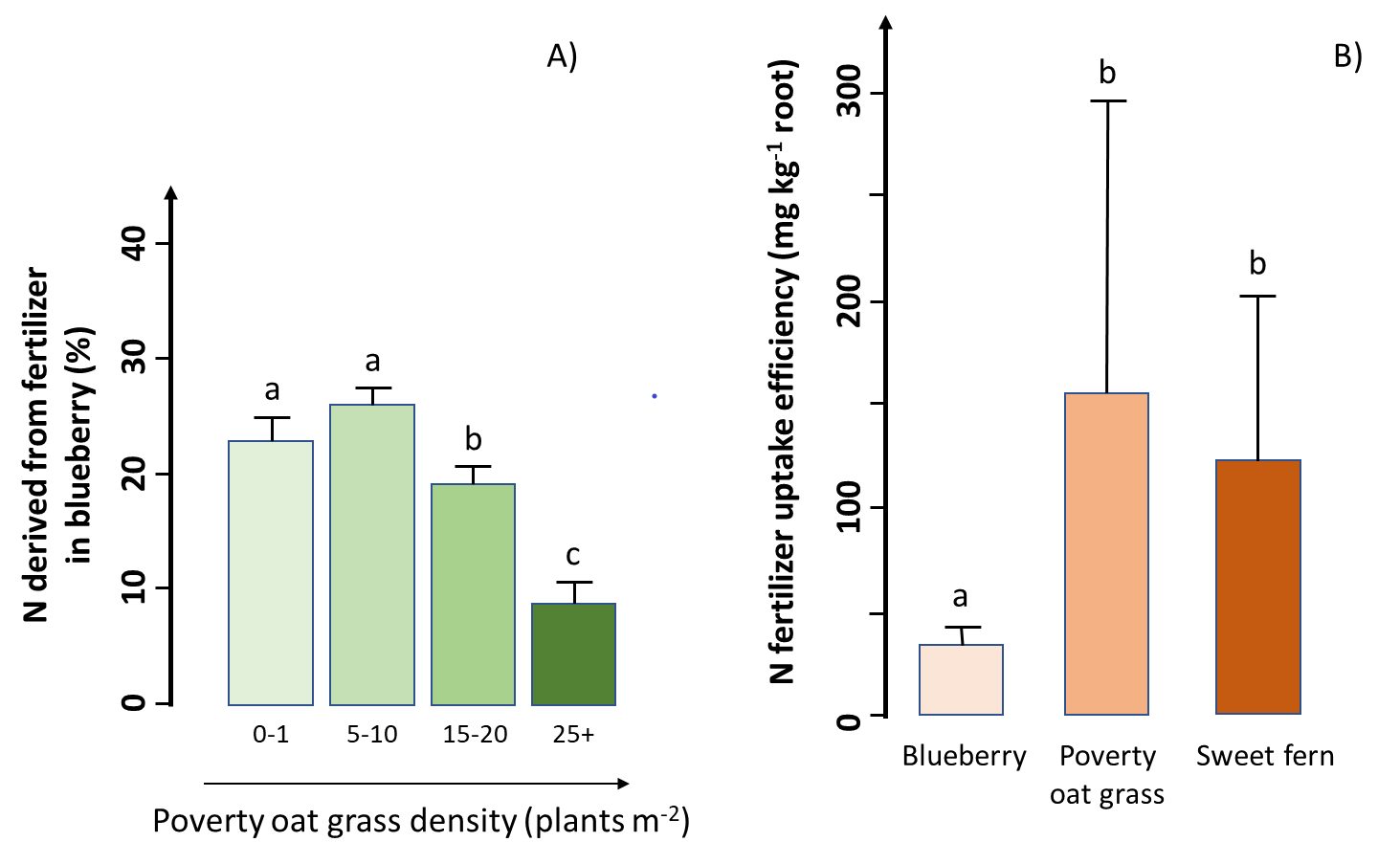
Researchers conducted a study in a lowbush blueberry (Vaccinium angustifolium Aiton) commercial field of the Lac Saint-Jean region, Québec, Canada, to assess the efficacy of N fertilization in the presence of two common weed species (poverty oat grass and sweet fern). The researchers found a much higher aboveground biomass production and a larger amount of N in blueberry than in both weed species, indicating that blueberry captured most of the added N fertilizer. However, blueberry growth and fertilizer uptake decreased with increasing weed density (Figure A). When poverty oat grass density was more than 15 plants m-2, this weed captured about the same amount of fertilizer as blueberry despite a much smaller belowground biomass, resulting in a significant negative impact on blueberry production. In addition, the researchers found that fertilizer uptake efficiency (the fertilizer uptake per gram of root) was about four times lower in blueberry than in both weed species (Figure B). In other words, compared to the weeds, blueberry requires four times more belowground biomass to acquire the same amount of N from fertilizer.
The complete story is provided in Marty et al. (2019)
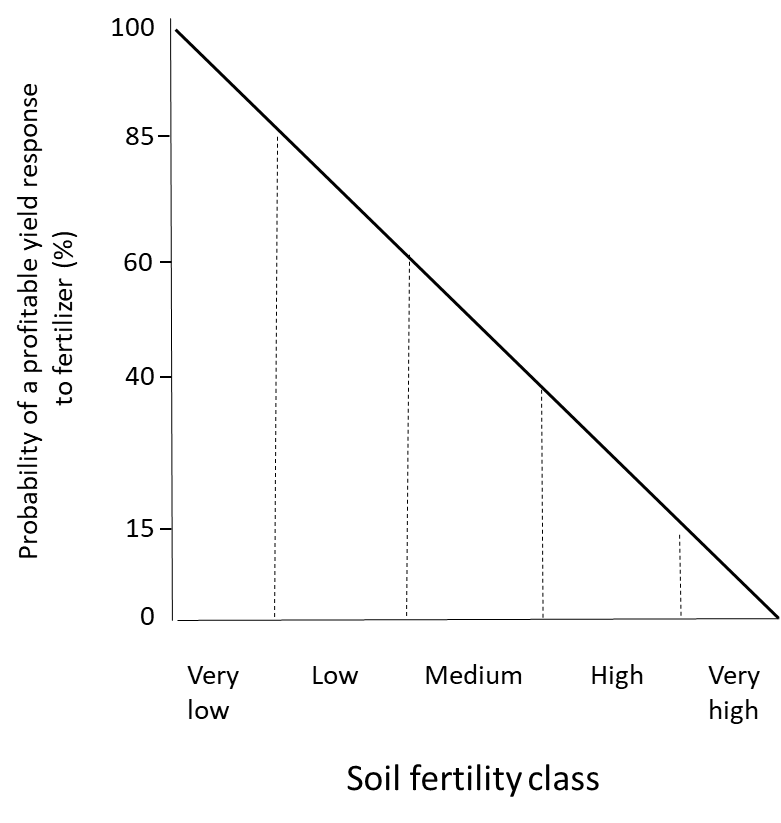
Since soil fertility testing quantifies the plant-available nutrient concentrations, it can be used to interpret the crop response to nutrient inputs. Soil fertility testing should be accompanied by measurements of the crop response to nutrient inputs in controlled environments such as growth chambers and greenhouses, and in the field. In soils with low to medium nutrient supply, crop growth will probably be improved by adding fertilizer. On the other hand, crops planted in soils with high to excessively high soil fertility rarely benefit from fertilizer application (Figure 7.5). Since fertilizers cost money to purchase, transport and apply to fields, it is not profitable to apply fertilizer when no yield improvement is expected, since this will not give an economic return on the investment.
Plant nutrition is determined by measuring the nutrient content of plants, either in a specific tissue or in the whole plant. When plants receive ample nutrients from the soil and fertilizer, if needed, their nutrient concentration will be in the sufficient range. When the soil nutrient supply is insufficient and no fertilizer is applied, the plant will be deficient in that nutrient. Plants that accumulate more nutrients than they need for their metabolic processes could have an excessively high or toxic level of nutrients (Figure 6). Nutrient toxicity is uncommon for macronutrients such as N, P, K, Ca, Mg and S because the active transport processes that transfers water-soluble nutrient ions through the endodermis and into the xylem are tightly regulated at a genetic level. Toxicity is sometimes observed when micronutrients such as B, Fe, Mn, Zn and Cu are applied at too-high rates to the foliage because these elements can damage the sensitive, photosynthetic cells in leaves. However, these micronutrients can be absorbed safely through the root system because plants have defense mechanisms that control the uptake, accumulation and translocation of micronutrients and other metals. This involves retaining the ion in root cells, where they are detoxified through complexation with amino acids, organic acids or metal-binding peptides and/or sequestered into vacuoles. These mechanisms will prevent micronutrient translocation to the sensitive leaf tissues.
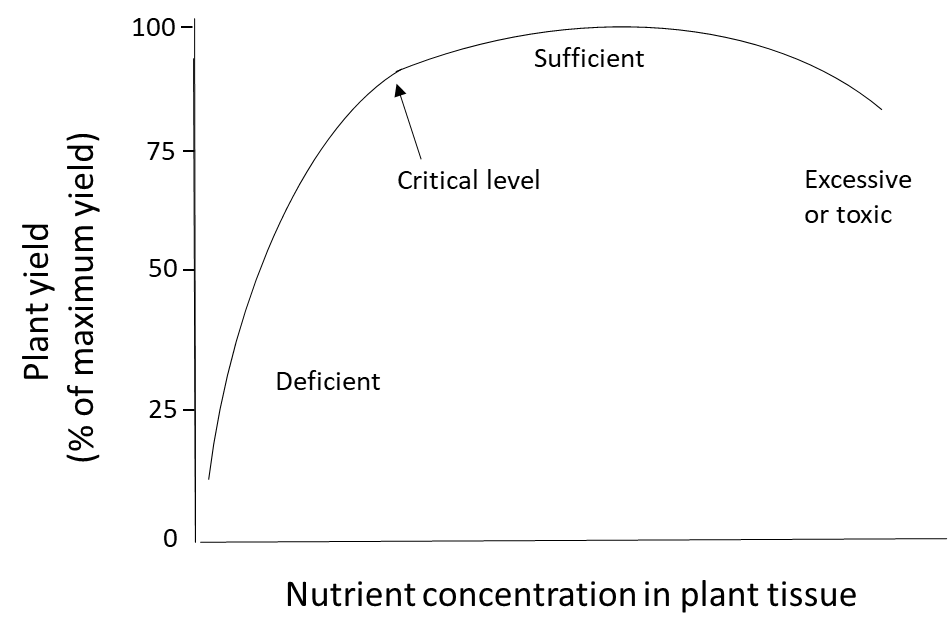
Nutrient requirements are known for all agricultural crops. Crops that have less than the critical nutrient level for development will not grow normally, will be stunted, and they may display deficiency symptoms such as chlorosis (yellowing of the leaves). This may be a sign that the plant lacked nutrients needed to produce chlorophyll, since a well-nourished plant will produce dark green leaves for photosynthesis. Even if there are no visible symptoms of a nutrient deficiency, inadequate plant nutrition can produce other undesirable outcomes:
- weak stems that are susceptible to lodging during windstorms, hailstorms and intense rainstorms;
- few flowers or inflorescences;
- seed abortion;
- poor-quality seeds (low protein content, lacking essential amino acids, low lipid content); and
- loss of marketable yield due to little seed/grain production, small fruits and tubers.
The best way to determine if crop growth is limited by lack of nutrients is to sample and analyze plant tissue. This involves taking a sample of the plant at an appropriate growth stage, generally during vegetative growth or in the early reproductive growth stage. The plant sample is then sent to a certified laboratory for analysis, and the results can be interpreted by checking reference tables that present the sufficient level of the macro- and micro-nutrients in a particular crop (Table 7.3). Since every crop has unique nutrient requirements at each of its growth stages, the reference table has to be specific for the crop species of interest. Furthermore, plant tissue analysis is done early in the growing season because the results may indicate that fertilizers should be applied to correct or prevent a nutrient deficiency. Timely analysis of plant tissue can guide the farmer to make an in-season nutrient application that will prevent yield losses. However, the diagnosis from a plant tissue test might arrive too late to take corrective action. For example, the crop may be too large for fertilizer applicators to enter the field. Another possibility is that the deficient nutrient is not mobile in the plant, meaning it is too late in the plant’s development to overcome the growth limitation associated with this nutrient deficiency. Therefore, soil fertility testing is the most important diagnostic tool to avoid a negative economic outcome that occurs when crops have inadequate nutrition during the growing season.
Table 7.3. Sufficiency ranges for macronutrients and micronutrients in corn plants, in relationship to corn growth stages (based on Jones, 2003)
| Element | V4-V6 stage (30 cm tall) |
V10 stage (prior to tasselling) |
R1 stage (initial silk) |
|---|---|---|---|
| Nitrogen (%) | 3.50-5.00 | 3.00-5.00 | 2.70-4.00 |
| Phosphorus (%) | 0.30-0.50 | 0.25-0.45 | 0.25-0.50 |
| Potassium (%) | 2.50-4.00 | 2.00-2.50 | 1.70-3.00 |
| Calcium (%) | 0.30-0.70 | 0.25-0.50 | 0.21-1.00 |
| Magnesium (%) | 0.15-0.45 | 0.13-0.30 | 0.20-1.00 |
| Sulphur (%) | 0.15-0.50 | 0.15-0.50 | 0.21-0.50 |
| Boron (mg kg-1) | 5-25 | 4-25 | 5-25 |
| Copper (mg kg-1) | 5-20 | 3-15 | 6-20 |
| Iron (mg kg-1) | 50-250 | 10-100 | 20-250 |
| Manganese (mg kg-1) | 20-300 | 15-300 | 20-200 |
| Molybdenum (mg kg-1) | 0.10-0.30 | 0.10-0.30 | 0.10-0.20 |
| Zinc (mg kg-1) | 20-60 | 15-60 | 25-100 |
FERTILIZERS AND SOIL AMENDMENTS
Fertilizer is any material that supplies water-soluble nutrient ions for crop production. Plants absorb nutrient ions from the soil pore water, without preference or discrimination among fertilizer sources. Therefore, any fertilizer that releases water-soluble nutrient ions could be beneficial to plants. Characteristics of some fertilizers that can increase the NH4+ and NO3– supply in soil are listed in Table 7.4.
Table 7.4. Characteristics of some nitrogen fertilizers that can supply NH4+ and NO3- for crops, based on IPNI (2012) and Munroe (2016)
| Fertilizer | Guaranteed analysis1 | Comments |
|---|---|---|
| Anhydrous ammonia | 82-0-0 | Applied as a compressed gas; requires special equipment and training |
| Calcium ammonium nitrate | 27.5-0-0 | Granular; water-soluble |
| Ammonium sulfate | 21-0-0 + 24% sulphur |
Granular; water-soluble |
| Urea | 46-0-0 | Granular; water-soluble Hydrolyzed by urease enzymes to release NH4+ |
| Urea ammonium nitrate | 28-0-0 | Solution containing 50% urea and 50% ammonium nitrate |
| Typical analysis2 | ||
| Liquid pig slurry | 2.2-2.7-1.9 | Solution (>90% water) with C:N ratio <5 |
| Dairy slurry | 1.2-1.8-2.9 | Solution (>90% water) with C:N ratio <10 |
| Chicken manure | 2.4-1.1-1.0 | Decomposed solid, C:N ratio <20 |
| Beef cattle manure | 0.8-1.8-2.6 | Partially-decomposed solid, C:N ratio <25 |
| 1Guaranteed analysis is based on the %N-%P2O5-%K2O in the fertilizer, on a dry weight basis. 2Typical analysis of manure is based on the %N-%P2O5-%K2O, on a wet weight basis |
||
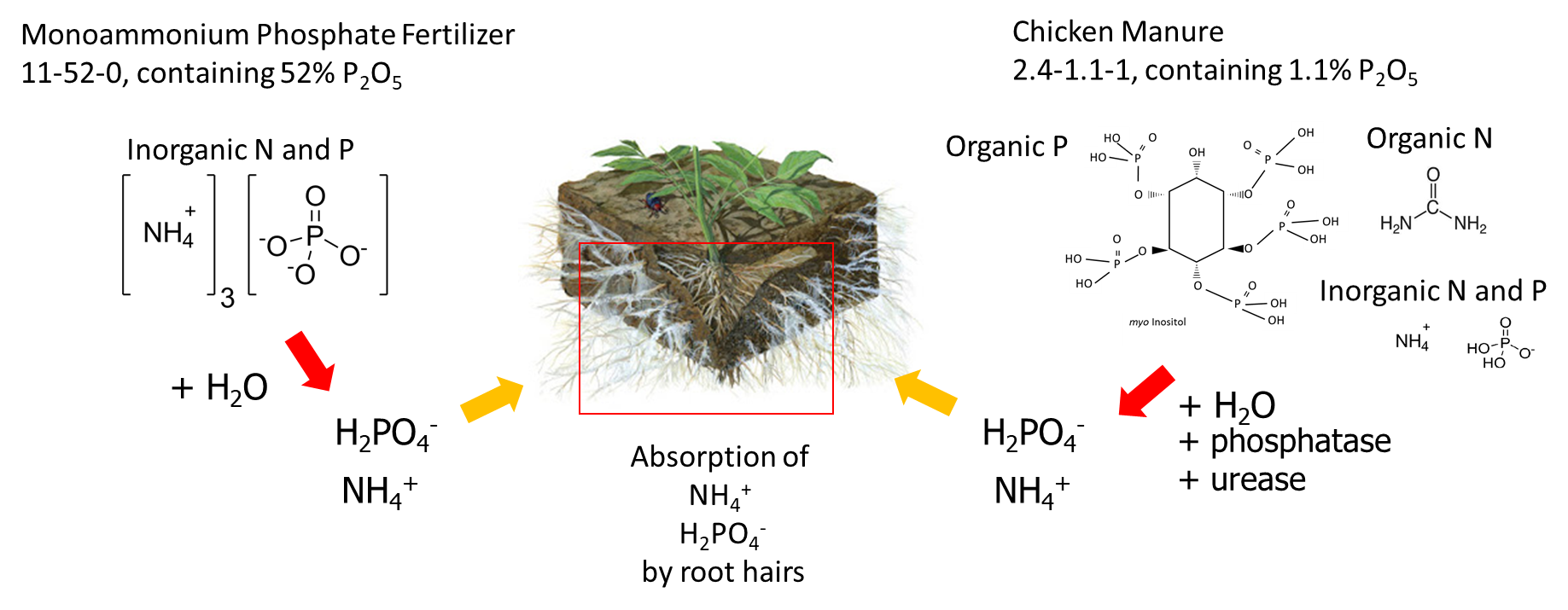
Fertilizer sources can be classified based on how they are manufactured, but from the perspective of plant nutrition, it is more interesting to consider how quickly the water-soluble nutrient ions are released into the soil pore water. For example, granular monoammonium phosphate purchased from a fertilizer company dissolves in soil water to release water-soluble NH4+ and H2PO4– ions. Another solid fertilizer is chicken manure, which contains about 4% total nitrogen and 0.5% total phosphorus (equivalent to 1.1% P2O5). The total nitrogen is composed of uric acid, protein and NH4+, and the total phosphorus contains orthophosphate compounds (50-75% of the total P) and organic phosphorus compounds such as myo-inositol (25-50% of the total P). Because chickens do not produce phosphatase enzymes in their digestive tract, only 10 to 30 per cent of the organic phosphorus in their food is absorbed by the animal and the undigested phosphorus is excreted. When chicken manure is applied to soil, the organic phosphorus compounds dissolve in soil water and are degraded by phosphatase enzymes that were produced by plant roots and soil microorganisms. Phosphatase enzyme hydrolyze organic phosphorus to release phosphate (HPO42-) ions into the soil pore water (Figure 7.7). The dissolution of granular monoammonium phosphate generally occurs faster than the hydrolysis of organic phosphorus compounds in chicken manure, which is why salt-based fertilizers are a ‘fast-release’ fertilizer and manure is considered a ‘slow-release’ fertilizer.
A soil amendment is a material that is added to improve the soil condition, but it does not necessarily provide nutrients for plant growth. Ground lime is often applied to acidic soils to increase the soil pH and reduce the concentration of Al3+ and Mn2+ in the soil pore water, since these elements can be damaging to crop roots. Neutralizing soil pH to around 6.0–7.0 provides more water-soluble NH4+, H2PO4– and K+, and increases the activity of soil bacteria, which are beneficial for plant growth and maintain soil structure. Other soil amendments include mulch and biochar. These carbon-rich materials are not expected to be a substantial source of plant-available nutrients, but they contain organic matter that can retain soil moisture and exchangeable ions, build soil structure and provide substrate for diverse heterotropic microorganisms. Since carbon-rich amendments stimulate microbial growth, they must be used sparingly to avoid the possibility that fast-growing microorganisms will immobilize nutrient ions from the soil pore water, thereby depriving plants of plant-available nutrients.
SOIL NUTRIENT CYCLES
Agricultural crops absorb nutrient ions from the soil pore water to produce edible biomass (e.g., grain, oilseed, forage, fruit and tubers) and non-edible biomass, some of which is harvested for animal bedding and for other purposes (i.e., second-generation biofuels are made from non-edible stems and leaves). Fertilizer addition will replenish the plant-available nutrients, but the nutrient ions from fertilizer will not necessarily remain in the soil pore water because they are subject to the biological, chemical and physical reactions of soil nutrient cycles, described below.
Nitrogen Cycling
Reactions that contribute plant-available nitrogen
The soil nitrogen cycle (Figure 7.8) is well studied, due to the central importance of nitrogen for chlorophyll production, Calvin cycle reactions that take place in chloroplasts during photosynthesis, and protein synthesis in plants. One way that agricultural crops can obtain nitrogen is through a symbiotic or associative relationships with N2-fixing prokaryotes. The best known of these are soil bacteria in the Rhizobium genera, but there are many others (Table 7.5). The N2-fixing prokaryotes possess the nitrogenase enzyme, which can transform atmospheric N2 into ammonia (NH3), the precursor for protein synthesis, through the reaction:
The nitrogenase complex reduces N2 to NH3, and it also reduces H+ to H2. The energy (ATP) for this reaction comes from the breakdown, via glycolysis, of photosynthates that are transferred from the host plant to the symbiont. The NH3 (g) product diffuses out of the bacteriod’s protoplasm into the root cortex, where it is protonated with 2 H+ ions, without additional energy, to yield:
The NH4+ then binds to glutamate to produce glutamine, or is transformed into ureides such as allantoin, allantoic acid or citrulline, before it is translocated from the root to other parts of the plant.

Legumes in the Fabaceae family form a symbiosis with N2-fixing bacteria, which inhabit nodules in their roots. The symbiosis is initiated when roots secrete flavonoids, which are chemo-attractants for the nodulating bacteria (rhizobia) to attach to the root hairs. The flavonoid signal activates the nodulation (nod) genes in the rhizobia, which synthesizes Nod factors that bind to surface protein receptors at the subapical root tip. This causes the root hair to curl and entrap the rhizobia, and the plasma membrane invaginates to form the infection thread, a tubular structure that extends from the root hair tip to lower cells of the root cortex. Rhizobia move through the infection thread and multiply, and root cortex cells proliferate into the nodule primordia, which will eventually become a fully-formed nodule enveloped in a membrane that prevents oxygen diffusion. This protective membrane prevents nitrogenase from being inactivated by oxygen. At this stage, the rhizobial bacteria become bacteroids capable of biological N2 fixation.
Although N2 is abundant (78% of the Earth’s atmosphere), the biological N2 fixation reaction is energetically demanding. In theory, 16 ATP are required to reduce one molecule of N2 to NH3 (g), but the actual energetic requirement is generally higher due to inefficiencies in the process. Therefore, biological N2 fixation is a strictly controlled process. All other available forms of nitrogen, such as NH4+, NO3– and amino acids, will inactivate the nitrogenase complex and inhibit the expression of the nif genes that encode for the nitrogenase components. In addition, the reaction products NH3 (g) and H2 also inhibit nitrogenase activity. Consequently, most legumes acquire 40-75% of their nitrogen requirement from biological N2 fixation, and the rest is absorbed as water-soluble NH4+ and NO3– from the soil pore water.
Associative N2-fixing bacteria live in the rhizosphere (in the soil pores and on soil particles close to plant roots) or as endophytes (within the plant tissues) of grasses such as sugarcane, rice, wheat, sorghum, maize and others. Some common genera are Azospirillum and Herbaspirillum, and there are many others (Table 7.5). These bacteria do not form nodules. Instead, they live on the root surface or within the intercellular space and xylem vessels of stems and leaves. They obtain energy from plant photosynthates and transfer a portion of the NH4+ generated from N2 fixation to the plant. There is considerable variability in their contribution to crop nitrogen requirements. In sugarcane, Gluconacetobacter diazotrophicus can supply 0-60% of the nitrogen requirement under field conditions. Cereals and oilseeds may obtain 1-25 kg N ha-1 y-1 from their associative N2-fixing bacteria. The rest of the nitrogen needed by these crops must be absorbed from the soil pore water as NH4+ and NO3– ions.
Table 7.5. Nitrogen-fixing prokatyotes that can supply nitrogen to plants. Adapted from Rascio and La Rocca (2013)
| Class | N2-fixing prokaryote | Host plant |
|---|---|---|
| Symbiotic | Allorhizobium spp. (fast-growing) Azorhizobium spp. (fast-growing) Bradyrhizobium spp. (slow-growing) Mesorhizobium spp. (intermediate-growing) Rhizobium spp. (fast-growing) Sinorhizobium spp. (fast-growing) |
Legumes (Fabaceae family) |
| Anabaena azollae (cyanobacteria) | Water fern (Azolla) | |
| Frankia spp. (actinobacteria) | Woody shrubs, e.g., in the families Betulaceae, Casuarinaceae and Myricaceae | |
| Associative | Azospirillum spp. Bacillus spp. Beijerinckia fluminense Derxia spp. Enterobacter spp. Erwinia spp. Gluconacetobacter diazotrophicus Herbaspirillum spp. |
Cereals (Poaceae family) Oilseeds (Brassicaceae family) |
Can You Dig It!
The importance of measuring soil N supply: A Prince Edward Island example
Nitrogen is a very dynamic nutrient—both critical to crop production and with the potential for significant environmental impact. Thus it is essential that we use nitrogen as effectively and efficiently as possible. In determining the need for nitrogen additions, we cannot forget to account for the nitrogen that will be supplied by the soil. In semi-arid and subhumid agroecosystems, such as those in the Canadian Prairies, residual nitrate-nitrogen (NO3-N) is an important source of plant N for the subsequent crop and should be measured in soil fertility tests and accounted for in fertilizer recommendations. In cool, humid agroecosystems such as those in Eastern Canada, overwinter loss of nitrogen is such that there is often limited carry over of nitrate (Zebarth et al., 2003). As a result, in-season nitrogen mineralization is an important crop input in both types of agroecosystems.
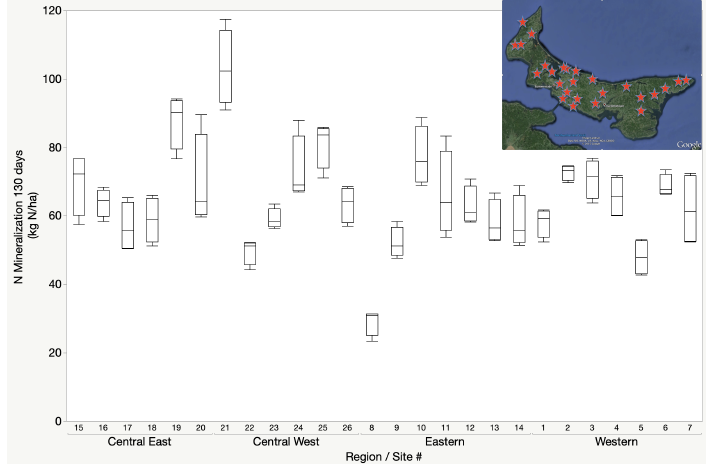
There are a variety of approaches to estimate the nitrogen mineralization potential that range from measures of the quality of the organic matter to the activity of the microbial community (Dessureault -Rompré et al., 2011). The variation in nitrogen mineralization potential can vary substantially as a result of soil moisture, temperature, soil type, cropping system or soil management. A survey of nitrogen mineralization potential of fields in three-year potato rotations in Prince Edward Island demonstrated that potential N mineralization over a 130-day growing period could range from 30–110 kg N ha-1. While there was on average greater N mineralization potential associated with central regions of the province, the greatest variation was from field to field, emphasizing the value of site-specific soil testing to quantify N mineralization potential.
Biological mineralization of proteins, also referred to as nitrogen mineralization, is the main source of NH4+ in unfertilized soil. This naturally-occurring process releases water-soluble NH4+ from decaying plant, animal and microbial residues, which were recently added to soil or are part of the soil organic matter. Soil bacteria and fungi produce protease and peptidase enzymes, as well as amino acid hydrolase enzymes that cleave the amine groups from amino acids and urease, which degrades the urea molecule. The general mineralization reaction is:
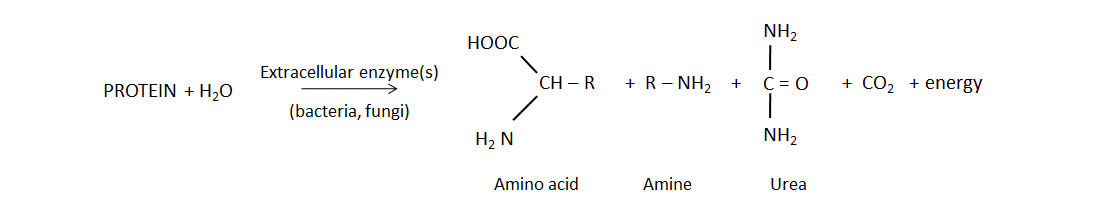 Nitrogen mineralization occurs more quickly with increasing soil temperature and is optimal when soil moisture reaches 60% water-filled pore space. Soil conditions that favour bacterial activity (pH from 6 to 7.5, low salinity) will stimulate mineralization, since bacteria have a faster generation time and greater capacity to produce protein-degrading enzymes than fungi.
Nitrogen mineralization occurs more quickly with increasing soil temperature and is optimal when soil moisture reaches 60% water-filled pore space. Soil conditions that favour bacterial activity (pH from 6 to 7.5, low salinity) will stimulate mineralization, since bacteria have a faster generation time and greater capacity to produce protein-degrading enzymes than fungi.
Nitrogen mineralization is also affected by the C:N ratio in organic residues. The C:N ratio is the mass of the total organic carbon divided by the mass of total nitrogen in an organic substance. Organic residues with a larger proportion of organic carbon provide substrates for heterotrophic microorganisms, which use carbon for metabolic processes and biomass production, and respire CO2. If their growth becomes limited by a lack of nitrogen in the organic residue, heterotrophic microorganisms will immobilize NH4+ and NO3– from the soil pore water. Organic residues with a C:N ratio > 20 such as woodchips, straw and grass-based residues tend to be rich in organic carbon and relatively impoverished in total nitrogen, which causes immobilization. Organic residues with a C:N ratio < 20 such as food-based compost and decomposed animal manure are relatively enriched in total nitrogen, so they will mineralize and increase the NH4+ concentration in soil pore water.
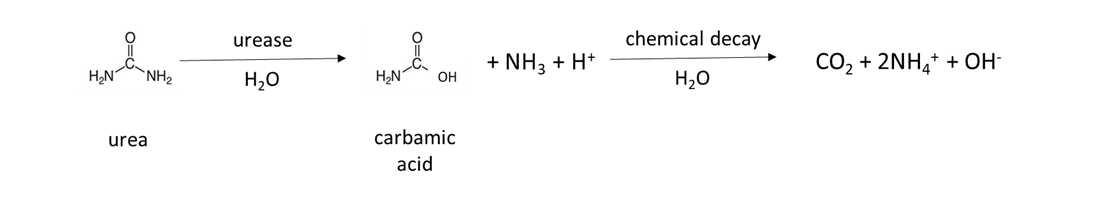
Many fertilizers are a source of NH4+ in soil. Granular fertilizer such as ammonium sulfate, (NH4)2SO4, is water-soluble and dissolves to release NH4+ (Table 7.4). Urea fertilizer is hydrolyzed by the urease enzyme, which is produced by naturally-occurring soil microorganisms and plants, to release NH4+:
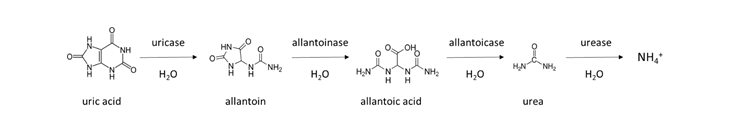
A similar reaction occurs in animal manure, which contains urea from animal urine. Chicken manure collected from laying facilities without bedding is enriched with urea-like molecules, due to the fact that 80% of the nitrogen excreted is in the form of uric acid. It takes several days to a week to transform uric acid to NH4+ in a warm, moist soil according to the general reaction:
Pig manure that is collected by spray-washing the barns and stored in a liquid form also has a high proportion of NH4+, due to urea hydrolysis and protein degradation that occurs in the manure storage. For example, liquid pig slurry contains >90% water, has a C:N ratio of 3, and the NH4+ form represents 75% of the total N content. The high proportion of readily-degradable uric acid in chicken manure, and the large amount of water-soluble NH4+ in liquid pig slurry, makes these manures “fast-release” nitrogen fertilizer, compared to animal manure mixed with bedding, which has a lower water content.
Ammonia oxidizers and nitrifiers are responsible for transforming the NH4+ to NO3–. The ammonia oxidizers are a diverse group of prokaryotes, including bacteria and archaea, which possess the amo and hao genes that encode for the enzymes that catalyze the following 2-step reaction:
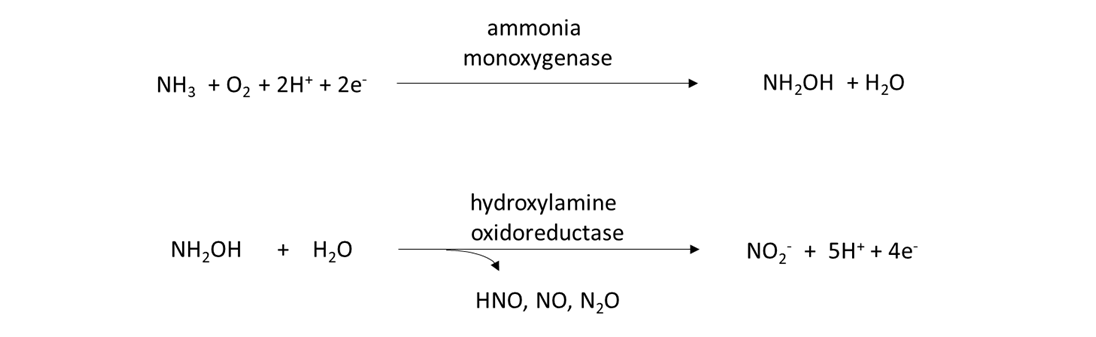
Nitrifiers are prokaryotes and eukaryotes that possess the nxr gene and are capable of converting nitrite (NO2–) into NO3–, as:
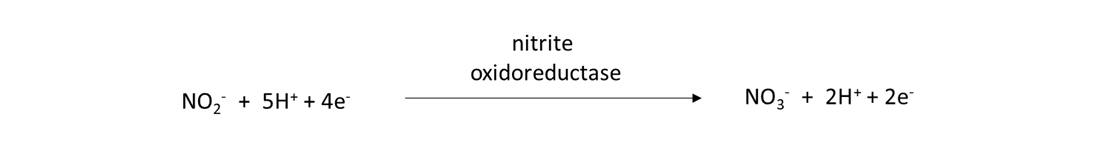
Ammonia oxidation and nitrification reactions occur quickly in field soils during the growing season. Consequently, NH4+ released from nitrogen mineralization or added in water-soluble fertilizer or animal manure will be transformed into NO3– within days to weeks. This understanding becomes important when deciding when to apply fertilizer, because we would like crops to absorb most of the water-soluble NH4– and NO3– from fertilizer as quickly as possible after the fertilizer application. Otherwise, reactions in the nitrogen cycle will remove these nutrient ions from the soil pore water, thereby reducing the amount of plant-available nitrogen for crops and increasing the risk of environmental pollution.
Can You Dig It!
Soil mineral nitrogen as predicted by ion exchange membranes
Ionic exchange membranes (IEMs) and plant root simulators were developed to determine N availability for agricultural and environmental studies. Indeed, IEMs contain positively or negatively charged surface functional groups that adsorb ions, such as NH4+ and NO3–, by electrostatic attraction. To maintain the equilibrium between the soil solid phase and soil pore water, IEMs continually remove NH4+ and NO3– that is mineralized from organic N or desorbed from organo-mineral surfaces.
One way to evaluate the NH4+ and NO3– concentration is to bury IEMs in the field for a period of time that depends on the objectives. Alternatively, the NH4+ and NO3– concentration can be assessed in a standardized laboratory environment. Once removed from the soil, IEMs are collected, washed with distilled water in the field to remove adhering soil particles and placed in individual tubes containing either NaCl (anionic exchange membranes) or HCl (cationic exchange membranes), and analyzed in laboratory for NO3– and NH4+, respectively. Soil nitrate and ammonium fluxes (supply rates) measured by IEMs are reported in µg cm-2 d-1 (Ziadi et al., 1999).
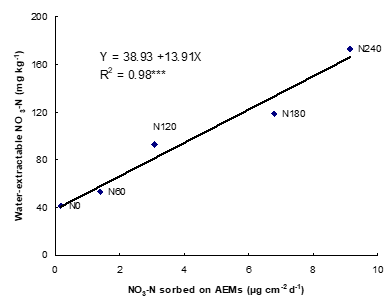
In general, there are significant correlations between IEMs and chemical extractions (Ziadi et al., 1999), and between crop N uptake and nitrates adsorbed on IEMs. Plant N uptake was more closely related to soil N as evaluated by IEMs than estimated by chemical extractions, suggesting that IEMs provide a better index of soil N availability than chemical extractions. Over all sites and treatments (mineral and organic N), NO3– and NH4+ adsorbed on IEMs increased with increasing N fertilizer rates. SoilNO3– and NH4+ adsorbed on IEMs varied largely with growing seasons and development stage of crop indicating the variation of soil N availability to plants. The in situ use of ionic exchange membranes as a soil testing procedure provide a more precise evaluation of N availability to different crops (forages, corn and potato) than standard methods. Based on its simplicity, rapidity, and low cost, IEMs have many practical advantages over chemical extractions for assessing soil N availability in eastern Canada.
Processes that deplete plant-available nitrogen
The NH4+ and NO3– ions in the soil pore water are removed through biological, chemical and physical processes. Nitrogen uptake by plants and immobilization by microorganisms is driven by nitrogen requirements for protein synthesis and formation of genetic material. In plants, nitrogen is a major component of proteins of the Calvin cycle (e.g., about 20-30% of the leaf nitrogen is found in the RuBisCO enzyme that initiates carbohydrate production), the thylakoids and the chlorophyll molecule and thus essential for CO2 fixation associated with photosynthesis.
Soil biological reactions that use NO2– and NO3– as electron acceptors are common. These reactions permit soil bacteria and fungi to acquire oxygen when the soil environment is anoxic. High soil moisture content can create anoxic conditions, as can intense biological activity that consumes oxygen and produce carbon dioxide, such as in the rhizosphere or in the detritusphere (i.e., around decomposing organic matter). Nitrifier-denitrification is one such reaction, and is expected when the soil moisture content is from 50 to 80% of water-filled pore space.
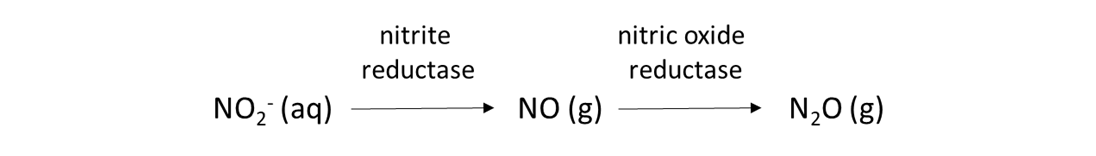
Denitrification is another biological reduction reaction that consumes NO3–, and it is expected when the soil moisture content exceeds 80% water-filled pore space.
A chemical reaction that depletes the NH4+ concentration is NH3 (g) volatilization, which occurs when NH4+ is deprotonated. During urea hydrolysis, this can be the result of carbonic acid formation:
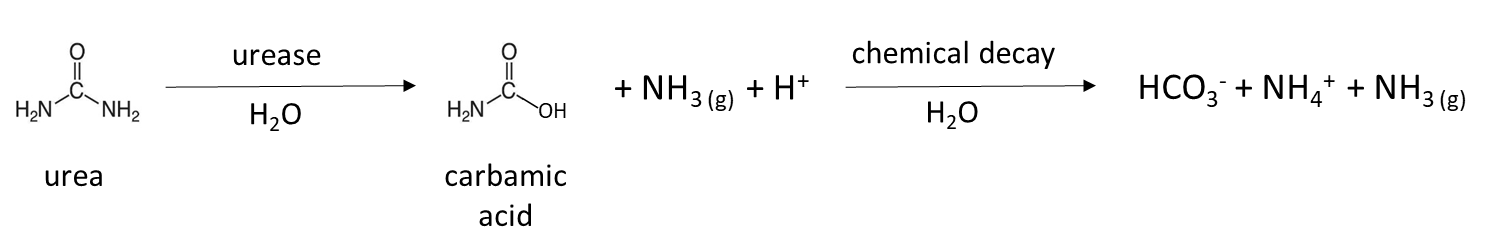
Animal manure can lose up to 50% of the total nitrogen excreted by the animal as NH3 (g) during manure storage and land application. Urea fertilizer is also susceptible to NH3 (g) loss, especially in alkaline soils that have an excess of OH– and HCO3– in the soil pore water, since H+ associates with these counter-anions rather than with NH3. Volatilization of NH3 (g) is undesirable because the transfer of gaseous nitrogen from agricultural fields to the atmosphere reduces plant-available nitrogen for the crop. Eventually, the NH3 (g) will return to the ground through wet and dry deposition, which results in the unintentional fertilization of non-agricultural land and aquatic systems.
Ammonium fixation is a chemical reaction that removes NH4+ from the soil pore water. This occurs when NH4+ adsorbs to exchange sites on organo-mineral surfaces. Ammonium fixation is greater in clayey soils, which have greater reactive surface area and more adsorption sites in the interlattices (2:1 clays only), than sandy soils. Due to its 1+ charge and small ionic radius, NH4+ is likely to bind to the interlattice, which reduces the amount of NH4+ in the soil pore water. This could account for a reserve of as much as 90–460 mg NH4+ kg-1 soil in fine-textured clays. In contrast, the NO3– molecule is loosely retained in the soil matrix due to its size and negative charge, and is more likely to leach through the soil profile and below the plant root zone. Nitrate leaching is affected by soil porosity and the water infiltration rate after snowmelt, rainfall and irrigation events. Finally, both NH4+ and NO3– are susceptible to loss from soil through erosion and surface runoff processes. Wind and water erode soil particles that contain adsorbed NH4+, while runoff water transports NO3– from saturated soils through overland flow.
Can You Dig It!
Optimizing the nitrogen application rate of animal manure

Balancing the rate of nitrogen addition with removal in the harvested crop over time prevents excessive accumulation of nitrate in the soil. Unused nitrate can slowly migrate down below the rooting zone over time with deep percolating water. In a trial in east-central Saskatchewan, liquid swine manure was added every year for eight years at a recommended agronomic rate of 37,000 L ha-1 yr-1, adding about 80 kg N ha-1 yr-1 which was about equal to the amount of N removed in the grain harvest each year. This rate resulted in little or no accumulation of nitrate in the soil profile, nor was there evidence of any deep leaching compared to an unfertilized control (lower right).
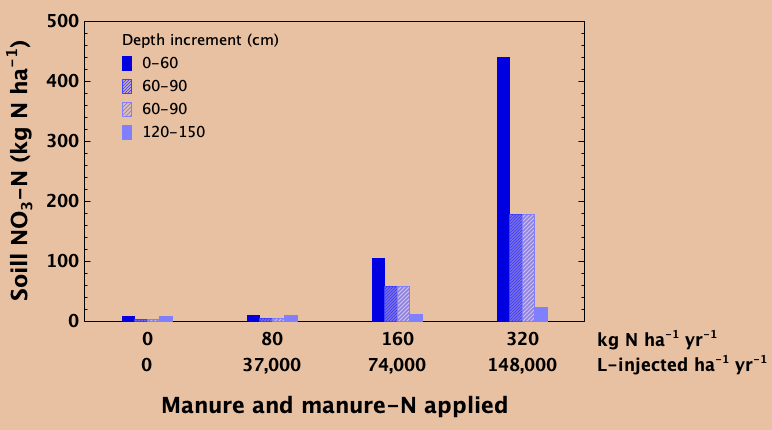
In the unfertilized control, crop production over the years was greatly reduced due to lack of available nitrogen while the agronomic rate produced good yields. At annual application rates that were double (74,000 L ha-1 yr-1) and especially quadruple (148,000 L ha-1 yr-1) the recommended rate, soil nitrate contents in the top 60cm as well as the depths beneath were elevated compared to the agronomic rate and the unfertilized control. Large amounts of unused nitrate that accumulate in the soil profile over time can slowly migrate to greater depths. If leached deep enough, it can become unreachable by plant roots and be a potential source of ground water contamination. With appropriate application rate that matches crop need and removal, this can be prevented.
Phosphorus Cycling
Reactions that contribute plant-available phosphorus
Plant-available phosphorus is derived from biological and geochemical processes (Figure 7.9). The biological source is organic phosphorus, which is hydrolyzed by phosphatase enzymes from plant roots and soil microorganisms. Organic phosphorus compounds are abundant in plant residues and in undigested plant materials. Inositols are major phosphate storage compounds that contribute to cell wall biosynthesis, cell-to-cell communication, storage and transport of plant hormones and other stress-related molecules, and osmoregulation. Degradation of myo-inositol releases water-soluble H2PO4–, as follows:
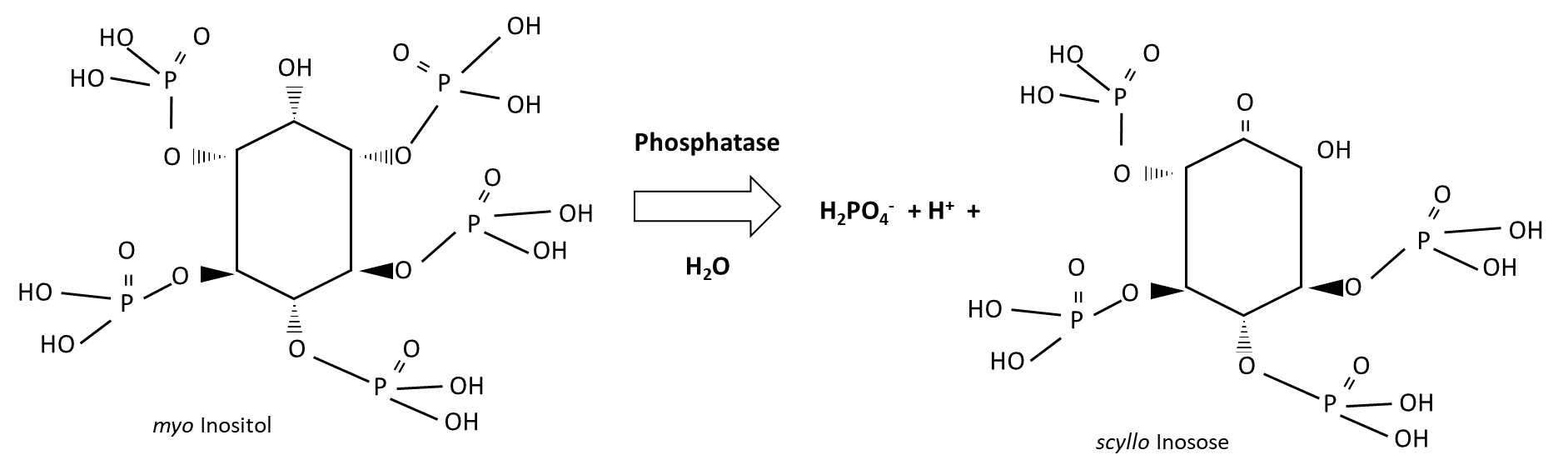
Primary minerals such as apatite undergo weathering to release H2PO4– into soil pore water. Other geochemical sources include H2PO4– that is desorbed from clay surfaces or dissolved from secondary minerals, the iron, aluminum, calcium and magnesium phosphates. Weathering and dissolution processes require water. The dissolution of calcium phosphates and magnesium phosphates are accelerated by the presence of carbonic acid (H2CO3 ↔ HCO3– + H+) and other acidic substances, however, iron phosphates and aluminum phosphates become less soluble in acidic soils. Inorganic acids that contribute to the solubilisation of H2PO4– from calcium phosphates and magnesium phosphates include H2SO4 and HCl. Organic acids produced by plant roots and soil microorganisms lower the pH in their surroundings, resulting in localized H2PO4– solubilization; in addition, organic acids can displace H2PO4– that is adsorbed onto clay and organo-mineral surfaces. Organic acids that are effective in this regard include acetic, lactic, malonic, malic, tartaric, oxalic, citric, ρ-hydrobenzoic and salicylic acids. Soil bacteria that produce organic acids, such as those from the genera Pseudomonas, Bacillus, Rhizobium and Burkholderia, can be isolated, cultivated and re-inoculated into soil to increase H2PO4– released via dissolution, but have not proven to be reliable at solubilizing H2PO4– under field conditions. Arbuscular mycorrhizal fungi also produce organic acids that increase water-soluble H2PO4–, which they then absorb through their extra-radial hyphae. Since arbuscular mycorrhizal fungi are plant symbionts, some of the H2PO4– acquired by the fungus can be transferred to the plant host through low and high affinity phosphorus transporters.
Long-term impact of tillage practices and phosphorus fertilization on soil phosphorus forms as determined by 31P nuclear magnetic resonance spectroscopy
Conservation tillage practices (minimum or no-till [NT]) are characterized by minimal soil disturbance and mixing and have been used more frequently in recent years to reduce off-site losses of nutrients associated with eroded particles, including phosphorus (P). However, by maintaining crop residues and fertilizers on the soil surface, the relatively immobile nutrients that do not readily move down the soil profile remain at or near the soil surface. Therefore, NT management systems often result in high concentrations of nutrients at the soil surface (0–5 cm) but sharply decreasing concentrations below this depth. The impact of tillage practices and P fertilization on soil P forms as determined using 31P nuclear magnetic resonance (NMR) spectroscopy was investigated under a long-term corn-soybean rotation experiment established in 1992 in Québec that compared NT with conventional (mouldboard plow; MP) tillage. The results showed that:
- total phosphorus varied with soil depth under the NT treatment— accumulating in the top unfertilized soil layer (0–5 cm) and in the 0–10 cm layer where P fertilizer was applied;
- pH, available P, and total carbon were significantly higher in the topsoil (0–5 cm) of the NT treatment than in the deeper layers (5–20 cm); total N also accumulated in the surface 10 cm;
- conversely, nutrient distributions were homogeneous throughout the soil profile under MP management—suggesting that stratification in NT results from the retention of crop residues at the soil surface;
- 31P-NMR spectra showed concomitant stratification of orthophosphate in NT plots that received 35 kg P ha-1;
- accumulation of scyllo-IP6 and nucleotides in the deeper layers was possibly due to their preferential movement through the soil column, whereas the pyrophosphate and DNA concentrations were greater at the surface (0–5 cm) soil layer than the deeper layers; and
- total C and N were similarly affected, suggesting that DNA was synthesized in greater amounts under NT owing to the higher organic matter accumulated at the soil surface in comparison to conventional tillage.
Overall, it appears that the accumulation of labile inorganic P at the surface of the NT soil may increase the potential for soluble inorganic P loss in surface runoff and lead to the loss of organic monoesters draining through different hydrological pathways. More information available at Abdi et al. (2014).
Reactions that deplete the plant-available phosphorus
The H2PO4– ions in the soil pore water are removed through biological, chemical and physical processes. Phosphorus uptake by plants and immobilization in microbial biomass are necessary for energy storage and transfer reactions, as well as the formation of nucleic acids and phospholipids.
Phosphorus retention reactions reduce the H2PO4– concentration in soil pore water because H2PO4– adsorbs to organo-mineral surfaces and to the surface of iron and aluminum sesquioxides, namely iron oxide (Fe2O3), iron hydroxide (Fe(OH)3) and aluminum hydroxide (Al(OH)3). These reactions are common in acidic soils, but occur in all soils to some extent. In general, soils with a higher clay content will adsorb more H2PO4–, particularly when they contain 2:1 expanding lattice clays such as smectite. The hydrated sesquioxides of iron and aluminum bind H2PO4– tightly and render it unavailable for plant uptake. In addition, H2PO4– precipitates with Fe, Al and Ca present in the soil pore water. This produces precipitates such as Fe(OH)2H2PO4 and the variscite-like compound, Al(OH)2H2PO4 in acidic soils. In neutral to alkaline soils, the H2PO4– precipitates with Ca to form slightly soluble dicalcium phosphate dihydrate, CaHPO4. Further reaction with Ca(OH)2 and CaCO3 in alkaline soils leads to the formation of insoluble hydroxylapatite (e.g., [Ca3(PO4)2]3•CaOH2) or insoluble carbonated calcium apatitie (e.g., carbonato-apatite; [Ca3(PO4)2]3•CaCO3).
The phosphorus saturation concept is another way to describe the capacity of soil to retain chemically-bound H2PO4–. Soil is shaken with an extracting solution such as Mehlich 3 solution, the amount of extractable P and Al is measured, and the ratio is calculated as:
(1)
Soil with a high degree of phosphorus saturation has elevated H2PO4–, relative to the amount of extractable Al that could bind with H2PO4–. Therefore, more water-soluble H2PO4– remains in the soil pore water and could leach after snowmelt, rainfall and irrigation events. In addition, erosion of soil particles that contain adsorbed H2PO4– will transport particulate P, while surface runoff will transport the dissolved phosphorus compounds at the soil surface through overland flow. The magnitude of phosphorus loss through these pathways depends upon the concentration of water soluble H2PO4–and particulate P in the surface soil, as well as the amount of leaching and severity of surface erosion processes.
Sulphur Cycling
Reactions that contribute plant-available sulphur
Plant-available sulphur can originate from weathering of parent material, atmospheric deposition, dissolution of gypsum (CaSO4) and mineralization of organic matter (Figure 7.10). Plutonic rocks such as pyrite contain elemental sulphur (So) that is oxidized to SO42- during the weathering process. Most oxidation reactions are mediated by autotrophic fungi and bacteria, such as Thiobacillus, which is abundant in the rhizosphere.

The conversion of So to SO42- is extremely variable in soils, due to natural variation in the distribution of sulphur oxidizing microorganisms. In general, sulphur oxidation reactions occur rapidly in warmer soils with moisture content with around 60% water-filled pore space and soil pH from 6-7.5. In addition, SO42- is released from the dissolution of hydrated salts in soil, such as CaSO4 • 2H2O and MgSO4 • 7 H2O.
Sulphur enters soil from atmospheric sources through dry and wet deposition. Several natural phenomena emit sulphur to the atmosphere, including particulate sulphur compounds that come from weathering of continental crusts (dust) and sea spray. Small particles of sulphur are transported by wind and deposited onto the surface of soil or vegetation. Gaseous emissions of sulphur dioxide, hydrogen sulfide, dimethyl sulfide and other gases from volcanic eruptions, swamps and coastal wetlands are another source. In the atmosphere, these gases tend to be oxidized to sulphuric acid and aerosol sulfate, and then return to the ground in rainfall and snow. These biogenic sources of sulphur account for <10 kg S ha-1 y-1 in terrestrial ecosystems. Since the Industrial Revolution, the anthropogenic sources of sulphur make a significant contribution to the soil sulphur cycle, and could exceed >50 kg S ha-1 y-1 in agricultural and forested lands in the proximity of industrial activities. Combustion of fossil fuels, biomass burning, waste incineration and the use of explosives all emit sulphur dioxide and therefore contribute through wet deposition to the sulphur content in soil. However, a reduction in air pollution has lowered the amount of sulphur released from these sources.
Organic sulphur is mineralized by soil enzymes – sulfatases – of plant and microbial origin to release SO42-, as illustrated below:
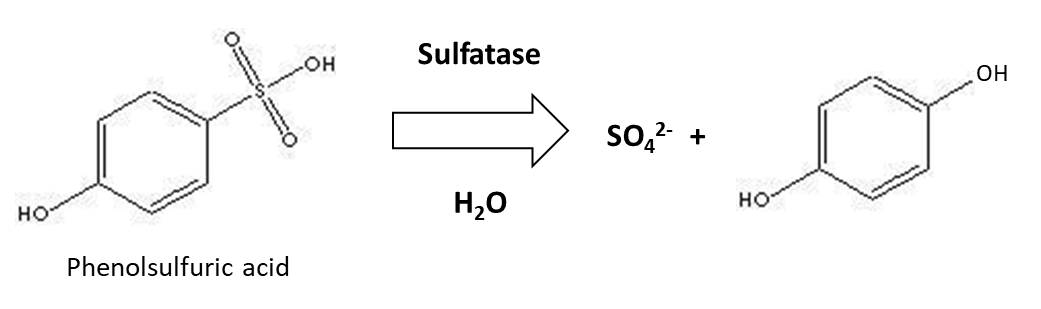
Like other mineralization reactions, sulphur mineralization increases with higher soil temperatures and is optimal when soil moisture content is around 60% water-filled pore space and soil pH is near neutral. Sulphur mineralization is higher around plant roots, which produce sulfatase. Also, plants absorb some of the SO42- in soil solution, which could limit the amount of SO42- available for microorganisms and therefore induce them to synthesize sulfatase. When fresh plant or animal residues are added to soil, the organic carbon to organic sulphur (C:S) ratio of the material will determine its susceptibility to mineralization. Organic residues with a C:S ratio <200 are mineralized readily and release SO42- into soil solution. However, organic residues with a C:S ratio >400 that have a low sulphur content could lead to SO42- immobilization by microorganisms, to meet their metabolic requirements.
Reactions that deplete the plant-available sulphur
The SO42- ions in soil pore water are removed through biological, chemical and physical processes. Biological uptake of SO42- by plants and microorganisms is necessary for the synthesis of essential amino acids (methionine, cysteine, homocysteine, and taurine), for production of glucosinolates, vitamins, as an enzyme cofactor and for chlorophyll production. In anoxic soils, sulfur electron acceptors gain electrons and decrease their oxidation state, so SO42- will be reduced to elemental sulphur So and then to S2- by sulphur-reducing bacteria in the phylum Firmicutes, including the genera Desulfotomaculum, Desulfosporomusa, and Desulfosporosinus.
Several chemical reactions reduce the SO42- concentration in soil pore water. In calcareous soils, the SO42- can be co-precipitated with CaCO3 to form a CaCO3 • CaSO4 complex. This accounts for about 10% of the total sulphur in calcareous soils. Second, SO42- binds to anion exchange sites on organo-mineral surfaces. However, SO42- is not as tightly bound to anion exchange sites as HPO42- and H2PO4–. Consequently, SO42- is susceptible to leaching and may be lost through erosion and runoff processes.
Potassium, Calcium, and Magnesium
The mineral elements—potassium, calcium, and magnesium—cycle through geochemical processes (Figure 7.11). Although plants and other organisms require substantial amounts of potassium, calcium and magnesium, these elements function in their ionic forms within biological tissues. For instance, potassium exists predominately as K+ in the vascular solution of plant tissues and in the cytoplasm of plant cells. It is translocated along with water in living plants. It may exist between layers of organic compounds such as polysaccharides, proteins and lipids, but it does not bind covalently to those substances. Leaching of K+ and other mineral elements such as Ca2+ and Mg2+ occurs when rainfall comes into contact with plant and animal residues, before biological decomposition begins.
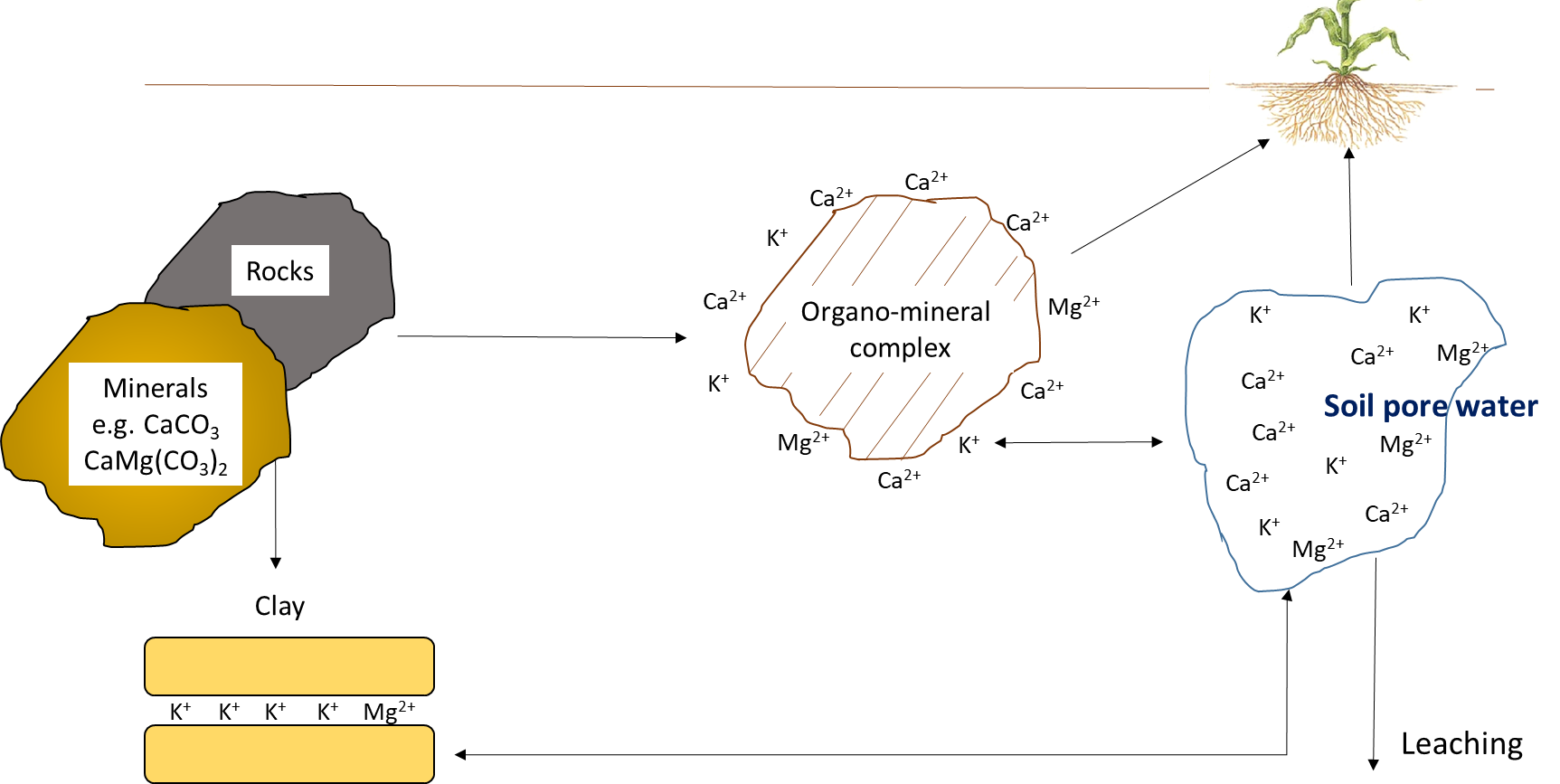
Reactions that contribute plant-available potassium, calcium and magnesium
The amount of K+, Ca2+ and Mg2+ in the soil pore water and on the cation exchange sites depends on the nature and weathering of the parent material. Organic residues contribute minor amounts of water-soluble K+, Ca2+ and Mg2+, compared to parent material. Potassium originates from primary minerals, such as feldspar, microcline, mica and biotite, and can also be added as fertilizer.
Soil Fertility Response to Intensive Crop Nutrition

From 2002 to 2006, a corn-soybean rotation in a grower’s field in south-central Ontario was monitored to assess changes in response to intensive management. The trial consisted of seven management combinations; three varying K rates at the grower’s level of inputs, and four at a more intensive input level—varying both K rate and zone placement.
The soil is a London loam with good drainage. Initial soil fertility levels were 8 ppm Olsen P (low), and 107 ppm ammonium-acetate extractable K (medium). Five treatments used the conservation tillage practice of the grower: fall chisel plow with spring secondary tillage. The other two treatments used fall zone tillage followed by spring zone tillage. Each treatment plot consisted of a strip eight rows wide that ran the full length (300 to 450 m) of the field (see photograph at right).
Yields averaged over the five years increased modestly in response to both increased K rates and management intensity. No combination of input intensity and K rate was more economically viable than the grower’s current level of management. There was no evidence of a greater requirement for K with higher input intensity.
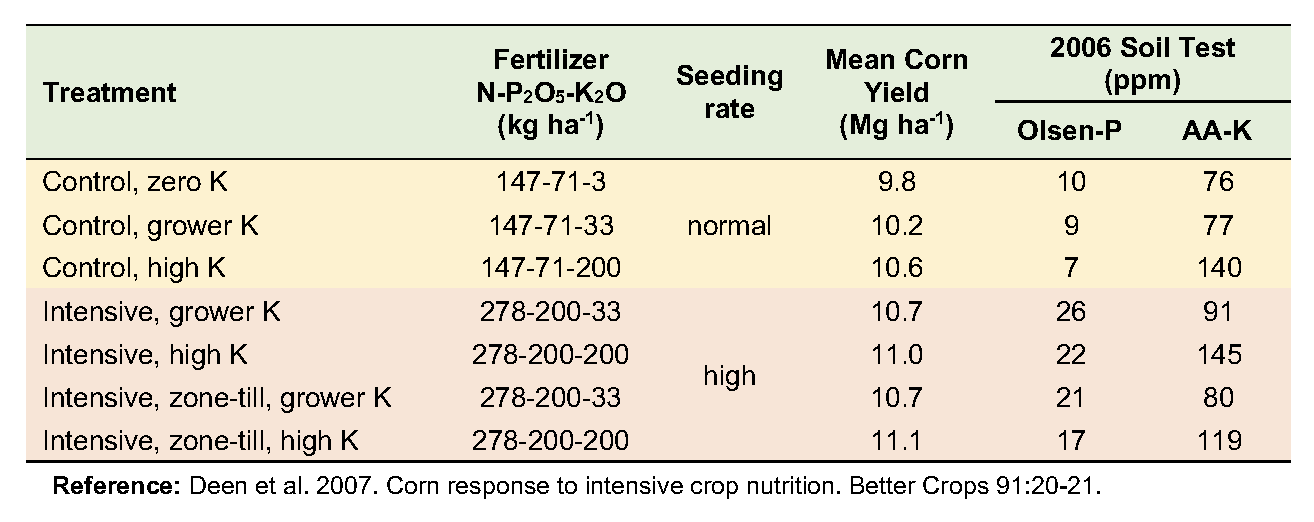 Soil test levels changed little where P and K inputs were at the grower’s rates but increased significantly where higher rates were applied. Grower rates resulted in net surpluses of P and net deficits of K, though the changes were not large enough to influence soil test levels. At the high rate of each nutrient, however, surpluses of P and K were large enough to substantially increase soil test levels for both nutrients. This work demonstrates that economically optimum crop yields require sufficient nutrient inputs to maintain soil fertility.
Soil test levels changed little where P and K inputs were at the grower’s rates but increased significantly where higher rates were applied. Grower rates resulted in net surpluses of P and net deficits of K, though the changes were not large enough to influence soil test levels. At the high rate of each nutrient, however, surpluses of P and K were large enough to substantially increase soil test levels for both nutrients. This work demonstrates that economically optimum crop yields require sufficient nutrient inputs to maintain soil fertility.
Calcium is released from weathering of primary minerals such as anorthite (CaAl2Si2O3), followed by pyroxenes and amphiboles, with smaller amounts of Ca coming from the weathering of biotite and apatite. Substantial amounts of Ca2+ are released from the dissolution of secondary minerals such as limestone (CaCO3), dolomitic limestone (CaMg(CO3)2), and gypsum (CaSO4⋅2H2O), all of which are major sources of Ca2+ in Canadian soils.
Magnesium in soils originates from rocks such as biotite, dolomite, hornblende, olivene and serpentine. Magnesium is often a substituted cation in clay minerals, such as chlorite, illite, montomorillonite and vermiculite. Weathering of rocks and clays releases Mg2+ into soil pore water, as does dissolution of secondary Mg minerals such as dolomitic limestone (CaMg(CO3)2), epsomite (MgSO4⋅7H2O) and bloedite (Na2Mg(SO4)3⋅4H2O).
There is a chemical equilibrium between the amount of K+, Ca2+ and Mg2+ in soil pore water and that on cation exchange sites. Smaller ions, particularly H+, can replace these mineral elements on exchange sites, leading to desorption of K+, Ca2+ and Mg2+ into soil pore water. The balance between exchangeable and soluble mineral ions is the key determinant of plant-available K+, Ca2+ and Mg2+ concentrations. Since pH affects the charge on organo-mineral surfaces (i.e., neutral to alkaline soils have more negatively charged surfaces than acidic soils), the exchange capacity is related to soil pH, clay and organic matter content.
Reactions that deplete plant-available potassium, calcium and magnesium
The K+, Ca2+ and Mg2+ ions in soil pore water are removed through biological, chemical and physical processes. Biological uptake of K+ is essential for enzyme activation (more than 80 enzymes require K+ as a cofactor), transport processes and osmotic regulation. The major functions of Ca2+ in plants are for maintenance of cell membranes, as well as cell division and elongation. An essential component in chlorophyll, Mg2+ is also a structural component in ribosomes and responsible for transfer reactions involving phosphate-reactive groups.
Adsorption reactions are the dominant chemical process that removes K+, Ca2+ and Mg2+ from the soil pore water. Adsorption can occur on the outer surfaces (“o” position), inner surfaces (“i” position) and edge (“e” positions) of clay minerals. Furthermore, these ions can be adsorbed in the interlattice of 2:1 clays in mica, illite and vermiculite. Due to its 1+ charge and small ionic radius, K+ tends to bind in the interlattice whereas Ca2+ and Mg2+ remain on outer surfaces of clay minerals. This reduces the amount of K+ in the soil pore water due to potassium fixation, which can account for up to 20% of the total K contained in soils with 2:1 clay minerals. The K+, Ca2+ and Mg2+ ions in soil pore water move out of the topsoil by leaching. This is associated with anion leaching, since anions that move through the soil pore water are accompanied by cations to maintain electrical neutrality. Erosion of clay-rich particles is another way that exchangeable K+, Ca2+ and Mg2+ ions are depleted from the topsoil.
Micronutrient Elements
Weathering is the primary source of most micronutrients, including iron, copper, manganese, zinc, nickel, boron and molybdenum. Plant-available chlorine may be dissolved from salts such as NaCl and KCl, or it may enter the soil in rainfall and irrigation water. Micronutrients are generally concentrated in the vascular solution and cytoplasm of plants, so they are present in organic residues and released during the initial physical fragmentation of decomposing organic residues. Consequently, plant and animal residues are a naturally-occurring source of essential micronutrients. Each micronutrient has specific functions in plants, listed in Table 7.6.
Table 7.6. Functions of micronutrients in plants, according to Jones (2003)
| Element | Functions |
|---|---|
| Iron | Fe-containing proteins, including heme proteins and Fe-S proteins, are important for photosynthesis, chlorophyll production and ribonucleic acid synthesis. Cytochromes are Fe-containing heme proteins, and ferredoxin is the most well-known Fe-S protein. |
| Manganese | Important in redox processes, including electron transport in photosynthesis and detoxification of oxygen-free radicals. An enzyme cofactor for biosynthesis of secondary metabolites such as phenolics, lignins and flavonoids. |
| Copper | Cu-containing enzymes react with molecular O2 and are important in photosynthesis, respiration, detoxification of superoxide radicals and lignification reactions. |
| Zinc | An enzyme activator that improves binding of enzymes and substrates involved in carbohydrate metabolism, protein synthesis and detoxification of superoxide radicals (O2-) to protect membrane lipids and proteins against oxidation. |
| Nickel | Required for the proper functioning of the urease enzyme that catalyses the reaction CO(NH2)2 + H2O → 2 NH3 + CO2. Any crop that produces ureides requires Ni. |
| Boron | Forms stable complexes with organic compounds that contributes to cell wall formation and stabilization, lignification and xylem differentiation. |
| Molybdate | Involved in electron transfer reactions. Mo-containing enzymes catalyse biological N2 fixation, and are responsible for pollen formation in plants. |
| Chloride | Mobile anion essential for osmoregulation (e.g., cell elongation, stomatal opening) and charge compensation (e.g., balance charges between cations and anions in the cytoplasm). Essential co-factor for photosynthesis. |
Plant uptake of micronutrients is improved at higher temperatures and in plants with prolific root systems, since greater evapotranspiration will move the water-soluble forms of micronutrients to the root surface, where they can be absorbed. Soil moisture has to be sufficient to maintain micronutrient ions in the soil pore water, but not so high as to create anoxic conditions that affect ion absorption by roots. For instance, anoxic conditions prevent uptake of Fe2+ by roots, but may increase absorption of toxic Mn2+ ions. Soil pH is often critical. The amount of plant-available iron, copper, manganese, zinc and nickel declines as the pH increases to the neutral and alkaline range. For example, iron deficiency can occur in plants growing in calcareous soils with pH >7.5. Plants that are adapted to this environment will respond by altering root morphology to increase the growth of root hair, release protons to acidify the rhizosphere and make Fe3+ more soluble, reduce the Fe3+ to Fe2+ with a plasma membrane-associated Fe3+ chelate reductase enzyme, and transport Fe2+ across the plasma membrane with an iron regulated transporter. Adapted plants in the Poaceae family have a different strategy, which involves chelation of Fe3+ by the phytosiderophores before the Fe3+ soluble complex is absorbed by the root.
Plants need far less of the micronutrient elements than macronutrients (Table 7.1). Soil reserves are often sufficient to meet plant requirements, particularly when in soil with pH 6–7 and when adapted cultivars are planted. We can improve micronutrient nutrition by adding fertilizer containing the deficient micronutrient(s) to the soil or directly to the plant foliage. With foliar application, a small amount of micronutrient fertilizer is sprayed onto the foliage in a solution with an adjuvant (to improve the adhesion of fertilizer solution to the leaf surface) during the plant’s vegetative growth or early reproductive growth stages. Water-soluble micronutrients such as H3BO3, Cu2+, Mn2+, MoO4– and Zn2+ and soluble chelates are absorbed through pores in the leaf and move through the plant’s vascular system to organs and tissues where they are required. Foliar fertilization is advantageous because it delivers a small amount of micronutrients and avoids adsorption and precipitation reactions that occur in soil. Due to the sensitivity of photosynthetic cells in leaves, the application rate and timing of micronutrients to foliage must be selected with care.
Non-essential Elements
Non-essential elements fall into two classes: (1) elements that are beneficial for some, but not all plants, and (2) elements that are not needed for plant growth. Elements that stimulate the growth of some plants include silicon, sodium and selenium. Silicon is beneficial for rice, sugarcane and other grass species. It accumulates preferentially in the stem tissues and appears to have a role in maintaining the structural integrity of the vascular system, which is required to transport water, nutrients, carbohydrates and other substances throughout the plant. Higher silicon concentrations are associated with greater drought tolerance. Sodium fertilization can improve the growth of some C4 plants, celery, spinach, sugar beet and turnips. Selenium appears to have general benefits related to oxidative stress tolerance and greater resistance to tissue damage caused by pathogens and herbivores.
Some elements can absorbed in quantities that are toxic to plants. In acidic soils with water-soluble Al3+ levels of 10 to 20 mg kg-1 or more, plants can be experience aluminum toxicity. The decline in plant root functions is related to oxidative damage of cell membranes, and less cell division and elongation at the growing tips due to Al3+ replacement of Ca2+. In sensitive plants, the presence of Al3+ in leaf tissues may reduce stomatal conductance and chlorophyll content, which reduces the photosynthesis rate. Plants grown in acidic soils are also prone to manganese toxicity when they absorb more Mn2+ than required for metabolic functions, resulting in oxidative stress and destruction of indole-3-acetic acid (auxin), an important plant growth hormone. Manganese toxicity symptoms are expected to be more severe in anoxic soils because oxygen limitation will reduce several low-solubility compounds, such as MnO2, Mn2O3 and Mn3O4, into Mn2+. Liming acidic soils with CaCO3 or another neutralizing substance that raises the soil pH will increase quantity of oxides and hydroxides, which form precipitates with Al3+ and Mn2+. Since precipitated aluminum oxides/hydroxides and manganese oxides are not water-soluble, the risk of plant toxicity diminishes with increasing soil pH. Installing drainage systems that increase the soil oxygen level should reduce Mn2+ toxicity in plants.
In salt-affected soils, an abundance of sodium (Na+), SO42- or Cl– creates salt stress for plants. Due to osmosis, root cells tend to lose water when the Na+, SO42- and Cl– levels are greater outside the root than inside the root. This can lead to physiological disorders within roots and other plant tissues, such as ion toxicity, altered metabolic processes, oxidative stress, membrane disruption, and less cell division and expansion. Consequently, plants require more water to grow in salt-affected soils. Transpiration is responsible for the movement of water and the accumulation of water-soluble salts near the root. Evaporation also causes salt movement into topsoil, particularly in semi-arid and arid climates where annual water loss is greater than annual precipitation. Soil management can be effective in removing excess Na+ and Cl– from the root zone. Sprinkler irrigation combined with improved drainage could leach these salts through the soil profile and away from plant roots. Salt-tolerant crops should be planted in salt-affected soils.
STRATEGIES FOR MANAGING SOIL NUTRIENTS
Soil fertility on farms cannot be managed without knowledge of the actual fertility status, based on observations of plant growth, yield measurements, plant tissue analysis and soil fertility testing in each field. A diagnostic evaluation of soil fertility also requires information about the amount of crop residues left in the field after the harvest, how much and how often agricultural lime and other soil amendments are spread on fields, and how much fertilizer is applied each year. We should also know the crop rotation, tillage, irrigation and drainage conditions in each field. This information can explain why soil organic matter and nutrient levels are increasing, stable or declining across the farm.
Although it is tempting to believe that we should always try to increase the soil organic matter and nutrient levels, this is not always achievable or desirable. There are physical limits to the amount of organic matter that can be retained in soil, related to climatic conditions (primarily temperature and rainfall) that determine the crop residues remaining after harvest, soil microbial activity and soil texture. Increasing the concentration of water-soluble nutrients in soils with high or excessively high fertility could increase the risk of nutrient loss to the environment through biological reactions (e.g., nitrogen loss in gaseous forms such as NH3, N2O and N2) and via leaching and erosion, which transport water-soluble NO3– and H2PO4– and particulate-associated nutrients to downstream environments. Consequently, we must use fertilizers and other amendments prudently and judiciously.
The value of measuring residual soil nitrate to assess nitrogen use efficiency
Residual soil nitrate at the end of the growing season is N that was not used by the crop. Thus, measuring how much nitrate remains in the soil profile following harvest provides a means of assessing how effectively we have managed N sources. In semi-arid and subhumid climates, this test is used to adjust the N fertilizer requirements for the next growing season. However, in cool humid agroecosystems where there is a high potential for non-growing season loss of nitrate, the residual soil nitrate test is an indicator of the risk of nitrate loss into aquatic systems. In Agriculture and Agri-Food Canada’s Agri-environmental Indicator series (Clearwater et al., 2016) the difference between N inputs and N outputs is referred to as Residual Soil Nitrogen (RSN) and has been estimated for the agricultural regions of Canada (see Figure below).
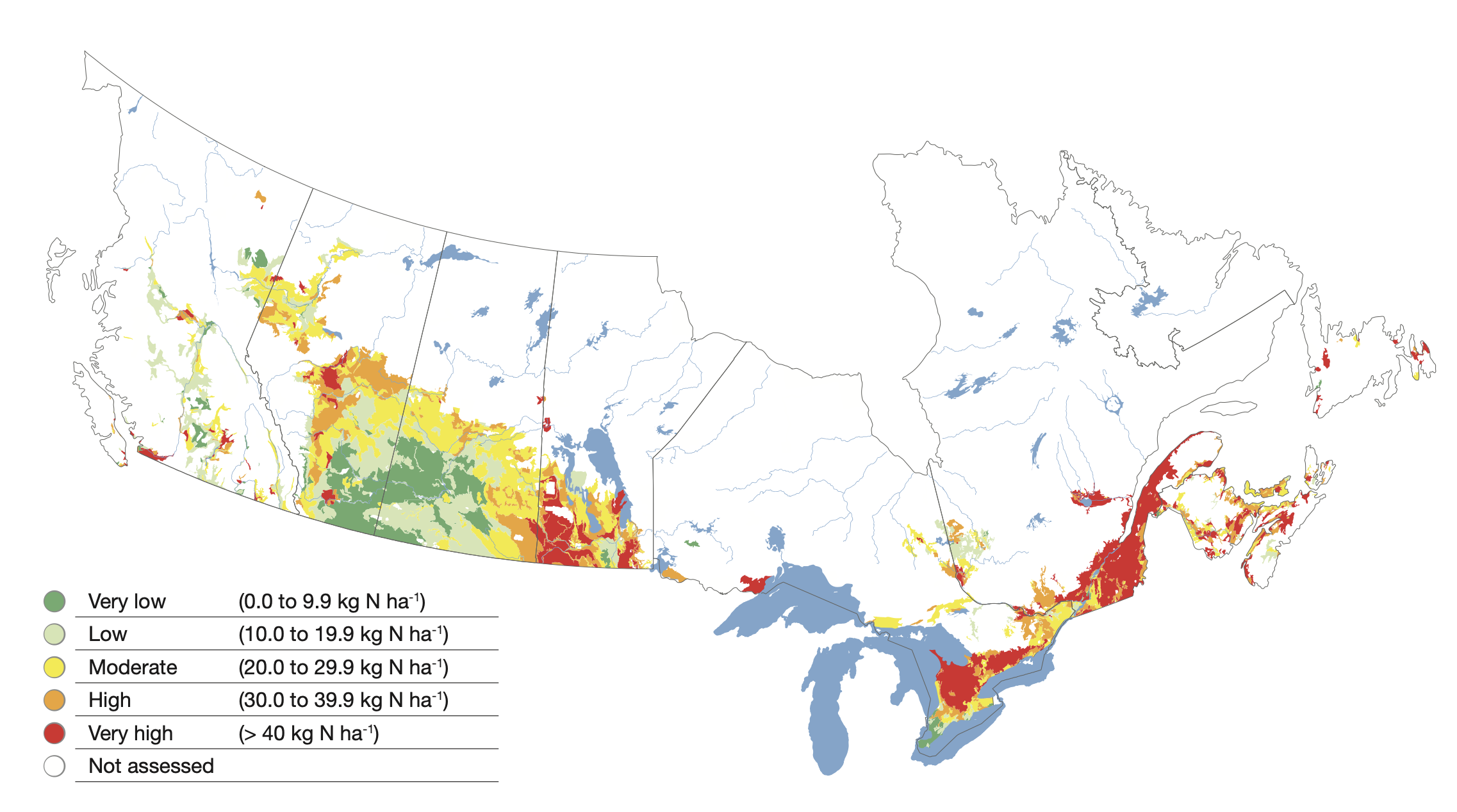
This indicator points to potential for a large amount of nitrogen remaining in the soil in the fall after harvest in Eastern Canada with typical values exceeding 40 kg N ha-1. Recent surveys of soil nitrate—measured to a depth of 45 cm—in Prince Edward Island suggest this estimate may indeed be conservative for potato production in that province with values averaging over 60 kg N ha-1. The implementation of 4R practices reduced RSN in potato production in PEI by as much as 30%.
Since crops are the intended beneficiary of nutrient in agricultural lands, fertilizer should be applied in a way that optimizes nutrient acquisition by the crop. The 4R nutrient stewardship approach is a decision-making framework that guides farms in selecting the ‘right’ actions related to fertilizer use, and is summarized below:
- Choose the right source of fertilizer that will release water-soluble nutrients for the crop. The right source of fertilizer also depends on its availability and price. Fertilizers produced on farms, such as manure or compost, could be a readily-available, inexpensive fertilizer. Apply these fertilizers to meet crop nutrient requirements first, before purchasing commercial fertilizers that will supply the rest of the required nutrients. Is a fast-release fertilizer needed to overcome a crop nutrient deficiency? Consider a fertilizer source that dissolves in water. A controlled-release fertilizer, which is biologically or geochemically stabilized and releases water-soluble nutrients slowly, could be suitable for annual crops with a long growth period or perennial cropping systems.
- Choose the right rate of fertilizer that permits your crop to reach its yield potential. When selecting the right rate, account for residual nutrients from decomposing crop residues and animal manure that were applied in previous growing seasons. Applying more nutrients than the crop can use will not improve the yield, although it could make weeds grow better. From an economic perspective, it is better to apply a little less fertilizer than you need, particularly when the growing conditions are sub-optimal due to a cold spring, flooding or drought. Applying more fertilizer during a poor growing season will not make the crop grow better and is a waste of money.
- Choose the right time to apply fertilizer so that you can synchronize the supply of water-soluble nutrients with periods of high nutrient demand by the crop. Annual crops have an exponential growth phase for several weeks during their vegetative growth period, which slows down after floral initiation. In humid and irrigated soils, the best strategy is to apply fertilizer just before the exponential growth phase, so the plants will absorb nutrients efficiently. In some crops, fertilizer should be applied at the beginning of the reproductive stage, to promote the development of high-quality seed. However, applying required fertilizers at the beginning of the growing season is a better strategy in semi-arid and subhumid regions because nutrient release is slow when precipitation occurs unevenly during the vegetative growth and initial reproductive stages. Perennial crops can be fertilized before they renew their vegetative growth each year. Perennial forages (i.e., hayfields) that are harvested two, three or four times a year can be fertilized after each successive cut, before the vegetative regrowth begins.
- Choose the right place to apply fertilizer so that you can maximize uptake of water-soluble nutrients by the plant root. Precise placement is required to avoid direct contact of fertilizer salts with seeds and seedlings, but not so far that water-soluble nutrients leach from the root zone before they can be absorbed by root hairs. The most efficient methods for annual crops involve placing fertilizer in the planted row (i.e., between seeds or tubers) or beside the planted row so that transpiration aids the movement of nutrient ions through mass flow (e.g., NO3–, SO42-) and diffusion (e.g., H2PO4–, K+) processes. Furthermore, as fertilizers dissolve or mineralize, they will release soluble nutrients that are accessible to the proliferating root system. Seeds can also be treated with fertilizer, as long as the fertilizer has a low salt content so there is no osmotic stress during germination and early seedling development. Foliar application is a rapid way to deliver small quantities of some micronutrient fertilizers, but there is a fine line between a micronutrient deficiency and a micronutrient toxicity in leaf tissues.
The 4R nutrient stewardship principles are the fundamentals of nutrient management on farms, but must be combined with soil and water conservation practices to be effective. The 4R Plus framework that is used on modern farms connects 4R nutrient management and soil and water conservation practices. The goal of the 4R Plus approach is to provide nutrients when the crop needs them, to enhance soil fertility and to improve water quality. Soil and water conservation practices that can enhance soil fertility and nutrient management include:
- Reduce tillage to minimize soil disturbance and improve soil structure.
- Leave crop residue on the soil surface to protect it from the impact of rainfall.
- Grow cover crops to protect the soil and support the soil microbial community between cropping seasons.
- Apply adequate fertilizer and manure using the 4R principles of right source, right rate, right time and right place.
- Value manure applications, since manure can enhance soil biology and soil fertility.
- Control field traffic to reduce soil compaction.
- Extend crop rotations and include perennial crops to build soil organic matter.
- Establish contour buffer strips, field buffers and stream buffers to protect sensitive environments within the field and in downstream environments.
- Highly erodible areas can be planted with perennial crops or with trees, rather than annual crops.
- Use prescribed or rotational grazing to minimize soil erosion.
Impact of long-term tillage and synthetic fertilization on soil parameters and crop yields under a corn–soybean rotation in eastern Canada
In eastern Canada, the corn (Zea mays L.)–soybean [Glycine max (L.) Merr.] rotation represents a dominant cereal–legume association. Although the economic benefits of this rotation are recognized, research is required to evaluate its long-term impact on soil quality and crop productivity under varying management alternatives such as reduced tillage or varying rates of synthetic fertilization. Indeed, the fundamental role of long-term field experiments in agronomic research and development has been recognized for decades by the international scientific community as essential for assessing the effects of management practices on soil fertility, soil quality, and crop productivity.
In 1992, a long-term research experiment was established in southern Quebec on a clay loam to evaluate the effect of tillage [mouldboard plow (MP) vs. no-till (NT)], synthetic N (0, 80, and 160 kg N ha-1) and P fertilization (0, 17.5, and 35 kg P ha-1) on soil functioning and crop yields of a grain corn–soybean crop sequence. Synthetic fertilizers were applied only to the corn phase of the rotation, while tillage was performed each year.
Results obtained 12 to 20 years after initiation of the study indicated that NT treatment enhanced organic C accumulation, NO3-N, P and K availability, and microbial activity in the upper soil layer likely due to accumulation of crop residues at or near the soil surface. This stratification can benefit soil structure but might impact surface water quality over the long term. Conversely, the MP treatment was characterized by a more uniform distribution of organic C and nutrients to tillage depth and higher microbial activity near the bottom of the plow layer. Strong links were established between soil tests P (e.g. Mehlich-3 and Olsen P) and P balance, explaining how P can accumulate in time with continuous agricultural practices. Soil N2O emissions were increased by N fertilization in corn but not affected by tillage. Grain corn yield linearly responded to fertilizer N addition in all years but soybean yield was little affected by residual N. Except for soil P status, fertilizer P had limited impact on both crops on this site but lack of replenishment would eventually lead to soil P depletion. Overall, MP gave greater yields than CT in several years (see above Figure)—by an average of 10% in corn and 13% in soybean—and provided generally a better profitability. The lower performance of CT in this site compared with MP is probably because cooler and wetter soil conditions in spring due to surface crop residues.
Note: this site is still operating and other studies are under investigation. For more details see Ziadi et al. (2014).
In the future, soil fertility managers will rely more on precision agriculture technologies to achieve the 4R Plus goals. The greatest advantage of precision agriculture, from the perspective of managing nutrients, is that it allows for the development of spatially-explicit maps of the soil and hydrological conditions in agricultural fields. The multiple layers of information in these maps permit us to evaluate individual parameters, such as soil pH or the soil potassium concentration across a field, or we can evaluate the interactions between multiple parameters (e.g., are changes in soil pH associated with fluctuations in the water table level across the field?). Rather than relying on an average soil fertility value for a large field, we can observe gradients in soil fertility that exist across the field. Knowing the zones of low, medium and high fertility allows us to adjust fertilizer application rates and meet the needs of the crop growing in a particular zone, rather than applying a uniform rate of fertilizer across the field. Soil and hydrological maps can also identify the vulnerable areas in fields where leaching or erosion are more likely to occur. Hence, the farm that adopts precision agriculture technologies can save money by applying fertilizers precisely, where they are needed, and establish buffer zones in the sensitive areas of the field and at the interface between fields and non-agricultural land. Precision agriculture technologies are well aligned with the 4R Plus goals and should be of great benefit to manage soil fertility in the future.
SUMMARY
- Water-soluble nutrient ions in the soil pore water are absorbed through root hairs of plants.
- Plant-available nutrients in the soil pore water can be transformed into unavailable forms through biological reactions, such as plant uptake, immobilization and reduction. Chemical precipitation and adsorption reactions also remove plant-available nutrients from the soil pore water. Physical loss of plant-available nutrients occurs when soils are leached and eroded.
- Soil pore water is replenished with plant-available nutrients through mineralization, oxidation, weathering, dissolution and desorption reactions.
- Soil fertility testing describes the soil nutrient supply and is required to make fertilizer recommendations. Crop nutrition is explained by plant tissue analysis, which determines whether the crop has sufficient nutrients or is deficient in nutrients required to achieve the economically optimal yield.
- Inorganic and organic fertilizers can be applied to replenish the soil nutrient supply and improve crop nutrition. Dissolution of inorganic fertilizers releases water-soluble nutrient ions quickly, whereas organic fertilizers contain some organic compounds that must be mineralized to release water-soluble nutrient ions, making them a relatively slow-release fertilizer.
- The 4R Nutrient Stewardship approach to soil nutrient management focuses on selecting the right source of fertilizer, to be applied at the right rate, at the right time and in the right place to get the greatest percentage of fertilizer nutrients into crop biomass. Achieving a high efficiency of fertilizer nutrient use in a cost-effective way is the goal.
- Soil nutrients are susceptible to loss from the soil-plant system, so farms should be aware of the environmental implications of soil nutrient management.
- The “Can you dig it?” boxes provide examples of soil fertility testing and soil nutrient improvement in Canada.
SUGGESTED READING
International Plant Nutrition Institute. 2012. 4R plant nutrition: a manual for improving the management of plant nutrition. North American version. IPNI, Norcross, GA, USA.
Havlin, J.L., J.D. Beaton, S.L. Tisdale, and W.L. Nelson. 2014. Soil fertility and fertilizers: an introduction to nutrient management. 8th ed. Pearson Prentice Hall, Upper Saddle River, NJ.
Munroe, J. (ed). 2016. Soil fertility handbook. 3rd edn. Ontario Ministry of Agriculture, Food and Rural Affairs Publication 611. Queen’s Printer for Ontario, Toronto. http://www.omafra.gov.on.ca/english/crops/pub611/pub611.pdf
Reid, K. 2014. Improving your soil: A practical guide to soil management for the serious home gardener. Firefly Books Ltd., Richmond Hill, Ontario, 272 p.
STUDY QUESTIONS
- How do soil nutrients get absorbed by plants?
- Complete the following table.
Nutrient Macronutrient or Micronutrient? Source in soil? Role in plant nutrition N P Ca S Cu Fe - Give an example of a biological reaction that can deplete the amount of plant-available nitrogen in the soil pore water.
- What is a chemical reaction that reduce the amount of plant-available potassium in the soil pore water?
- Why does the application of a fertilizer containing NH3 tend to acidify the soil?
- Nitrogen is often the most limiting nutrient for agricultural crops to reach their yield potential, so is it a good idea to add excessively high rates of nitrogen fertilizer to agricultural land? Why or why not?
- What is the best diagnostic tool to determine the fertilizer requirements of crops: soil fertility testing or plant tissue analysis?
- Micronutrient fertilizers can be applied to crop foliage. What is the advantage of this fertilization method?
- What are some considerations to help you choose the right fertilizer source for your crop?
- What are suitable soil and water conservation practices to prevent nutrient loss through erosion?
REFERENCES
Abdi, D., Cade-Menun, B.J., Ziadi, N., and Parent, L.-É. 2014. Long-term impact of tillage practices and phosphorus fertilization on soil phosphorus forms as determined by 31P nuclear magnetic resonance spectroscopy. J. Environ. Qual. 43(4): 1431–1441.
Bender, R.R., J.W. Haegele, M.L. Ruffo, and F.E. Below. 2010. Nutrient uptake, partitioning, and remobilization in modern, transgenic insect-protected maize hybrids. Agron. J. 105(1): 161–170.
Carter, M.R. and Gregorich, E.G. 2008. Soil sampling and methods of analysis. 2nd ed. Canadian Society of Soil Science. CRC Press, Boca Raton, FL.
Centre de référence en agriculture et agroalimentaire du Québec. 2010. Guide de référence en fertilisation. 2e édn. Centre de référence en agriculture et agroalimentaire du Québec, Ste Foy, QC.
Chantigny, M.H., Angers, D.A., Morvan, T., and Pomar, C. 2004. Dynamics of pig slurry nitrogen in soil and plant as determined with N-15. Soil Sci. Soc. Am. J. 68(2): 637–643.
Deen, B., Lauzon, J., and Bruulsema, T. 2007. Corn response to intensive crop nutrition. Better Crops 91: 20–21. http://www.ipni.net/publication/bett...48874B7A852579 800081D5CE/$FILE/Better%20Crops%202007-2%20p20.pdf
Dessureault-Rompré, J., Zebarth, B.J., Chow, T.L., Burton, D.L., Sharifi, M., Georgallas, A., Porter, G., Moreau, G., Leclerc, Y., Arsenault, W.J., and Grant, C.A. 2011. Prediction of soil nitrogen supply in potato fields in a cool humid climate. Soil Sci. Soc. Am. J. 75(2): 626–637.
Drury, C.F., Yang, J., De Jong, R., Huffman, T., Reid, K., Yang, X., Bittman, S., and Desjardins, R. 2016. Residual Soil Nitrogen. Pages 114–120 In R.L. Clearwater, T. Martin, and T. Hoppe (Eds.) Environmental Sustainability of Canadian Agriculture: Agri-Environmental Indicators Report Series. Report #4. Ottawa, ON: Agriculture and Agri-Food Canada
Elbon A., and Whalen, J.K. 2015. Phosphorus supply to vegetable crops from vesicular arbuscular mycorrhizae: a review. Biol. Agric. Hort. 31(2): 73–90.
Eskin, N., Vessey, K., and Tian, L. 2014. Research progress and perspectives of nitrogen fixing bacterium, Gluconacetobacter diazotrophicus, in monocot plants. Int. J. Agron. 2014, 13 p. Article 208383. https://doi.org/10.1155/2014/208383
Evans, J.R. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78(1): 9–19.
Fleury, D. 2020. 4R nitrogen management for wheat and canola. Top Crop Manager 19 August 2020 https://www.topcropmanager.com/4r-ni...at-and-canola/
Follett, R.H., Murphy, L.S., and Donahue, R.L. 1981. Fertilizers and soil amendments. Prentice-Hall, Inc., Englewood Cliffs, NJ.
Havlin, J.L., Beaton, J.D., Tisdale, S.L., and Nelson, W.L. 2014. Soil fertility and fertilizers: an introduction to nutrient management. 8th ed. Pearson Prentice Hall, Upper Saddle River, NJ.
He, Z.Q., Honeycutt, C.W., Griffin, T.S., Cade-Menun, B.J., Pellechia, P.J., and Dou, Z.X. 2009. Phosphorus forms in conventional and organic dairy manure identified by solution and solid state P‐31 NMR spectroscopy. J. Environ. Qual. 38(5): 1909–1918.
International Plant Nutrition Institute. 2012. 4R plant nutrition: a manual for improving the management of plant nutrition. North American version. IPNI, Norcross, GA, USA.
Jones, J.B., Jr. 2003. Agronomic handbook: management of crops, soils and their fertility. CRC Press, Boca Raton, FL.
Karamanos, R.E. 2008. Nitrogen and phosphorus recommendations for Manitoba for today’s production costs. Presented at the 2008 Manitoba Agronomists Conference, Winnipeg, MB
http://umanitoba.ca/faculties/afs/MA...is%20Karamanos %20paper.pdf
Kour, D. Rana, K.L., Kaur, T., Yadav, N., Yadav, A.N., Kumar, M., Kumar, V., Dhaliwal, H.S., and Saxena, A.K. 2021. Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and -mobilizing microbes: A review. Pedosphere 31(1): 43–75.
Kumar, V., Vogelsang, L., Schmidt, R.R., Sharma, S.S., Seidel, T., and Dietz, K.-J. 2020. Remodeling of root growth under combined arsenic and hypoxia stress is linked to nutrient deprivation. Frontiers in Plant Science. 23 October 2020. https://doi.org/10.3389/fpls.2020.569687
Leytem, A.B., Smith, D.R., Applegate, T.J., and Thacker, P.A. 2006. The influence of manure phytic acid on phosphorus solubility in calcareous soils. Soil Sci. Soc. Am. J. 70(5): 1629–1638.
Marty, C., Lévesque, J.-A., Bradley, R.L., Lafond J., and Paré, M.C. 2019. Contrasting impacts of two weed species on lowbush blueberry fertilizer nitrogen uptake in a commercial field. PLoS ONE 14: e0215253.
Mehlich, A. 1984. Mehlich-3 soil test extractant: a modification of Mehlich-2 extractant. Commun. Soil Sci. Plant Anal. 15(12): 1409–1416.
Mortvedt, J.J., Cox, F.R., Shuman, L.M., and Welch, R.M. 1991. Micronutrients in agriculture. SSSA Book Series 4, American Society of Agronomy, Madison, WI.
Munroe, J. (ed). 2016. Soil fertility handbook. 3rd edn. Ontario Ministry of Agriculture, Food and Rural Affairs Publication 611. Queen’s Printer for Ontario, Toronto. http://www.omafra.gov.on.ca/english/...611/pub611.pdf
Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA). 2006. Nutrient management planning. Best Management Practices Series, Guelph, ON.
Rascio, N., and La Rocca, N. 2013. Biological nitrogen fixation. Reference Module in Earth Systems and Environmental Sciences. Elsevier, Amsterdam. doi:10.1016/B978-0-12-409548-9.00685-0
Reid, R., and Hayes, J. 2003. Mechanisms and control of nutrient uptake in plants. Int. Rev. Cytol. 29: 73–114. doi: 10.1016/s0074-7696(03)29003-3.
Ruiz Herrera, L.F., Shane, M.W., and López-Bucio, J. 2015. Nutritional regulation of root development. WIREs Dev. Biol. 4: 431–443. doi: 10.1002/wdev.183
Sims, J.T., Maguire, R.O., Leytem, A.B., Gartley, K.L., and Pautler, M.C.. 2002. Evaluation of Mehlich 3 as an agri-environmental soil phosphorus test for the Mid-Atlantic United States of America. Soil Sci. Soc. Am. J. 66(6): 2016–2032.
Stumborg, C., Schoenau, J.J., and Malhi, S.S. 2007. Nitrogen balance and accumulation pattern in three contrasting prairie soils receiving repeated applications of liquid swine and solid cattle manure. Nutr. Cycl. Agroecosyst. 78: 15–25.
Westerman, R.L. 1990. Soil testing and plant analysis. 3rd edn. SSSA Book Series 3, American Society of Agronomy, Madison, WI.
Whalen, J.K. 2014. Managing soil biota-mediated decomposition and nutrient mineralization in sustainable agroecosystems. Advances in Agriculture 2014: 13 p. Article 384604. doi: 10.1155/2014/384604
Whalen, J.K., Thomas, B.W. and Sharifi, M. 2019. Novel practices and smart technologies to maximize the nitrogen fertilizer value of manure for crop production in cold humid temperate regions. Adv. Agron. 153: 1–85.
Zebarth, B.J., Leclerc, Y., Moreau, G., Gareau, R., and Milburn, P.H. 2003. Soil inorganic nitrogen content in commercial potato fields in New Brunswick. Can. J. Soil Sci. 83(4): 425–429.
Ziadi, N., Angers, D.A., Gagnon, B., Lalande, R., Morel, C., Rochette, P., and Chantigny, M.H. 2014. Long-term tillage and synthetic fertilization affect soil functioning and crop yields in a corn–soybean rotation in eastern Canada. Can. J. Soil Sci. 94 (3): 365–376.
Ziadi, N., Simard, R.R., Allard, G., and Lafond, J. 1999. Field evaluation of anion exchange membranes as a N soil testing method for grasslands. Can. J. Soil Sci. 79(2): 281–294.
About the Authors
Joann K. Whalen, James McGill Professor at McGill University and an Adjunct Professor at Gansu Agricultural University and with the Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences

Joann received her B.Sc. (Agr.) from Dalhousie University, M.Sc. from McGill University, and Ph.D. from Ohio State University (USA). She is a professional agronomist (agronome) in Quebec, Canada. Her research focuses on soil fertility and soil ecology in agroecosystems. She has published more than 230 peer-reviewed scientific publications and supervised/co-supervised more than 70 students at the M.Sc., Ph.D. and postdoctoral levels. She teaches courses in soil fertility, nutrient management planning and environmental soil chemistry.
Joann is a Chief Editor for Soil Biology and Biochemistry, an academic journal that publishes original, scientifically challenging research articles of international significance that describe and explain biological processes occurring in soil. She is a Fellow of the Soil Science Society of America and of the Canadian Society of Soil Science. Joann is the first international scientist to be elected for a 3-year term as President of the American Society of Agronomy (2022-2024). She grew up in a farming community near Sussex, New Brunswick and has always been interested in the practical aspects of growing food. In her opinion, soil fertility specialists are the most broadly-trained agricultural scientists because they have to muster knowledge from so many fields – physics, hydrology, chemistry, biochemistry, plant biology, microbiology and genetics – to understand the complex and dynamic soil processes responsible for crop growth. To make sense of soil responses, we have to dig deeper, which comes quite naturally to a soil scientist!
Noura Ziadi, Research Scientist, Agriculture and Agri-Food Canada; Adjunct Professor Université Laval, the Graduate University of Chinese Academy of Science, Université du Québec en Abitibi-Témiscamingue

Noura is a research scientist with Agriculture and Agri-Food Canada in Quebec City. She holds a B.Sc. (Agronomy) from Tunisia and a M.Sc. and a Ph.D. (Soil Fertility and Plant Nutrition) from Université Laval, Quebec, Canada. Noura is also an adjunct professor at the Université Laval (2005-), the Graduate University of Chinese Academy of Science (2012-), and the Université du Québec en Abitibi-Témiscamingue (2016-).
Noura has a distinguished career in soil science especially soil fertility and plant nutrition and she’s conducting her research at the national and international scales (France, China, Switzerland, Finland, Tunisia, Iran, Saudi Arabia) within multi-disciplinary teams including government and university researchers and industry partners. Noura published more than 155 papers, five reviews and 11 book chapters including 37 papers with the Canadian Journal of Soil Science (CJSS). Noura joined the CSSS in 1994 and has served as Eastern Councillor (2008-2010), President-Elect (2017), President (2018) and Past-President (2019). Noura is an associate editor (AE) for the CJSS since 2011, has handled 107 manuscripts for this journal and was been recognized as a best AE in 2017. Noura has been recognized for her contributions to soil science and to the CSSS with a Fellow Award in 2015.
Jeff J. Schoenau, Professor and Saskatchewan Ministry of Agriculture Research Chair – Nutrient Cycling & Management, Department of Soil Science, University of Soil Science

Jeff is a professor of soil fertility and professional agrologist who works in the Department of Soil Science at the University of Saskatchewan. He holds the Saskatchewan Ministry of Agriculture Soil Nutrient Management Chair in the College of Agriculture and Bioresources, and is a fellow of the Agricultural Institute of Canada. He was born in Saskatchewan, completed his undergraduate and graduate degrees in the 1980’s in the College of Agriculture at the University of Saskatchewan and has worked there since. He also farms with his spouse Lynne near Central Butte, Saskatchewan. His research, teaching and extension activities deal with soil fertility and fertilizers, nutrient cycling, and soil management practices in prairie cropping systems.
Maxime C. Paré, Professor, Université du Québec à Chicoutimi

I teach soil science to undergraduate students, most of whom are enrolled in the biology program at UQAC. I teach soil science as an introductory class, and we look at soil chemistry, biology, nutrient cycling, and pedology. It is always interesting how initially most students view soil science negatively—I usually do a small survey during the first class of the semester—and how their views change over the semester as the students realize the importance of soil for biology, life, and the general well-being of the planet. I also include soil science in most of my field teaching activities. I like to show the links between spheres, such as how what we see when we look up, e.g., the traits of plants and trees, strongly influences what we find when we look down, i.e., in the soil, and vice versa. Finally, soil science allows me to do research and support many graduate students across a variety of research disciplines, from various agricultural domains (wild blueberry, haskap, potatoes, cash crops, etc.) to forestry and the environmental sciences. How ever could I be more stimulated?
David L. Burton, Professor, Department of Plant, Food, and Environmental Sciences, Faculty of Agriculture, Dalhousie University

Dr. David Burton is a Soil Scientist and Professor in the Department of Plant, Food, and Environment, Dalhousie University. His research examines the role of the soil environment in influencing the nature and extent of microbial metabolism in soil. His focus has been on processes in the cycling of nitrogen in soils and their implications for soil fertility and environmental impact. It is the aim of this work to better understand the factors that control soil microbial function and to use this information to developing resilient land management systems in response to a changing climate.
Tom Bruulsema, Chief Scientist with IPNI Canada

Since July 2019, Tom Bruulsema is Chief Scientist with Plant Nutrition Canada, providing support for the nutrient stewardship programs of Fertilizer Canada, The Fertilizer Institute, and the International Fertilizer Association. Prior to the dissolution of the larger IPNI, he was vice-president for the Institute’s programs in the Americas, and research globally. From 2015 to 2017 he led the Institute’s Phosphorus Program. Dr. Bruulsema has been recognized as a Fellow of the American Society of Agronomy, the Soil Science Society of America, and the Canadian Society of Agronomy. He was previously a research associate at the University of Minnesota (1994), and an agronomist with the Mennonite Central Committee in Bangladesh (1986-1990).


