6.4: Biogenous Sediments
- Page ID
- 45544
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Most non-microbial life in the oceans is sustained by the conversion of dissolved carbon dioxide to living organic matter by photosynthetic organisms. Phototrophy takes place only in the upper part of the water column (at most a few hundred meters deep) where sufficient light is present (Chap. 12, CC14). With only minor exceptions, the photosynthetic organisms of the oceans are small (less than about 2 mm), as are most of the animals that feed on them (Chap. 12). Very few of these organisms live out a full natural life span, because most are consumed by larger animals. The resulting fecal material is excreted in the form of fecal pellets. Fecal pellets, although small, are much larger than the microscopic organisms of which they are made up. Therefore, they tend to sink toward the seafloor relatively rapidly, as do bodies of larger animals that have not been ingested by another animal (CC4). Most life in the deep-ocean waters and on the seafloor is sustained by the rain of this organic-rich detritus (Chap. 13).
One species, a small planktonic tunicate called a “giant larvacean,” may contribute a substantial proportion of the total detritus that rains into the deep. The giant larvacean secretes an intricate netlike structure to capture its food. The larvacean lives inside the structure, which, consequently, has been called a “house.” When the web becomes clogged with particles, which happens about once a day, the larvacean simply releases the house and secretes a new one. The abandoned houses are large enough that they sink fairly rapidly, and this source may then contribute a substantial proportion of the detritus that reaches the seafloor, at least in some areas.
As detritus falls through the water column, it is either utilized by animals or decomposed by bacteria, archaea and fungi, and decomposition continues on the seafloor. In all but a few locations where the rain of detritus is extraordinarily heavy or where there is insufficient oxygen to support bacterial decomposition, the organic matter in detritus is essentially completely decomposed. Consequently, little organic matter is incorporated in the accumulating bottom sediment in most areas. In contrast, many marine species have hard parts that are not decomposed as they fall through the water column, and these materials constitute the overwhelming majority of inputs of biogenous particles to ocean sediment.
The hard parts, shells, or skeletons of marine organisms are either calcareous or siliceous. Calcareous organisms have hard parts of calcium carbonate (CaCO3); siliceous organisms have hard parts of silica in the form of opal, which has the same basic composition as glass (SiO2). Ocean surface waters are populated by very large numbers of calcareous and siliceous organisms, most of which are smaller than a few millimeters. Hence, a continuous rain of both calcareous and siliceous particles falls from the surface layers. This material does not rain uniformly on the sediment surface because marine productivity and types of organisms vary by region, and because calcium carbonate and silica dissolve in seawater at slow but variable rates.
The two major factors that determine the accumulation rate of biogenous sediment are the rate of production of biological particles in the overlying water column and the rate of decomposition or dissolution of these particles as they fall to the seafloor. The percentage of biogenous material in the sediment is determined by these two factors plus the rate of accumulation of other particles.
Regional Variations of Biogenous Particle Production
Chapters 12 and 13 examine the factors that determine primary productivity rates and the types of organisms that inhabit various regions of the oceans. The distributions of productivity, biomass, and dominant organism type (e.g., calcareous or siliceous) in the ecosystem are major factors in determining the nature of sediments that accumulate in any given area.
In high latitudes and areas of coastal upwelling (Chap. 13), siliceous diatoms are the dominant photosynthetic organisms (Fig. 6-6). Diatoms are among the largest of the phytoplankton (up to about 2 mm) and yield relatively large siliceous particles. At lower latitudes and in the open ocean, many of the dominant photosynthetic organisms have no hard parts. However, one group of very tiny photosynthetic organisms called coccolithophores (Fig. 6-7a) may grow in abundance. They are covered by a number of small calcareous plates that contribute extremely fine particles to the sediment. The white chalk cliffs of Dover in England consist primarily of coccolithophore plates (Fig. 6-7b).

Zooplankton with calcareous shells include foraminifera and pteropods (Fig. 6-8), many species of which are present throughout the oceans. Animals with siliceous hard parts are less common, but the microscopic radiolaria (Fig. 6-9) have intricate silica shells and are abundant in tropical waters that have high primary productivity.

Dissolution of Biogenous Particles
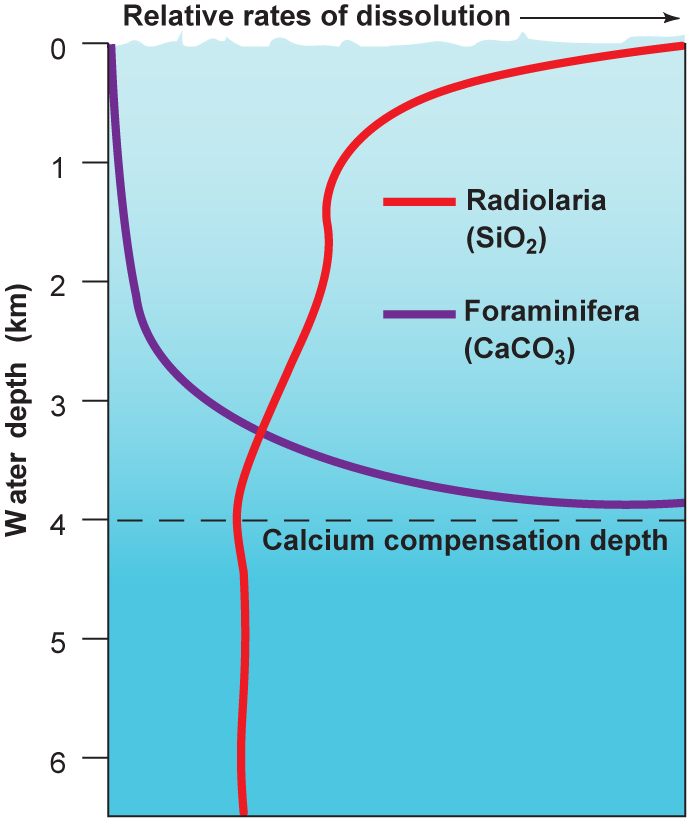
Seawater is undersaturated with silica. Therefore, siliceous particles dissolve as they fall through the water column to the seafloor. The rate of dissolution is very slow at all depths, but it decreases with depth throughout the upper 2 km of the water column (Fig. 6-10). Thin siliceous hard parts are almost always totally dissolved and reach the sediment only in areas where siliceous organisms grow in great abundance. For example, many radiolaria have thin, intricate shells that are dissolved relatively easily and reach the sediment in substantial quantities only in highly productive tropical waters where they abound.
The production of fecal pellets can enhance the accumulation of siliceous and calcareous particles. When packaged in relatively large fecal pellets, such particles fall more quickly to the seafloor and are partially protected from dissolution until the organic matter in the pellet disintegrates or is decomposed.
Many diatoms have a relatively thick siliceous frustule (hard part), much of which reaches the seafloor, where it continues to dissolve until buried by other particles. Diatom frustules are present in the sediment in locations where their production rate and the sedimentation rate are high.
Calcareous particles also dissolve in seawater, but their behavior is different and more complex than that of siliceous particles. In the upper water layers, where water temperatures are high and pressures low (Chap. 8), seawater is generally supersaturated with calcium carbonate. Consequently, calcareous shells dissolve only very slowly or not at all in near-surface waters (Fig. 6-10). The solubility of calcium carbonate increases with pressure and with decreasing temperature. Therefore, solubility tends to increase with depth.
Not all calcareous hard parts are made of the same mineral. Calcium carbonate shell material is of two distinct forms: calcite and aragonite. Some types of animals, including most foraminifera, have calcite shells, whereas others, such as pteropods, have aragonite shells. Aragonite dissolves much more readily in seawater than calcite. Therefore, pteropod shells are dissolved more completely at shallower depths than foraminiferal shells. Where pteropods are more abundant than foraminifera, shallow-water sediments can consist of predominantly pteropod particles. Sediments at intermediate depths are predominantly foraminiferal. Sediments at greater depths, where the dissolution rate is high, have almost no calcareous component (Fig. 6-11).

Carbonate Compensation Depth
Below a certain depth, seawater is undersaturated with respect to calcium carbonate, and calcareous debris starts to dissolve. Below this level, the degree of undersaturation and hence the dissolution rate of calcium carbonate increases with depth (Fig. 6-10). Eventually, at the carbonate compensation depth (CCD), the dissolution rate is fast enough to dissolve all of the calcium carbonate before it can be incorporated in the sediment.
The CCD depends not only on pressure and temperature, but also on other factors, especially the concentration of dissolved carbon dioxide. An increase in carbon dioxide concentration lowers the pH, making the water more acidic, and thus increases the solubility of calcium carbonate.
Carbon dioxide solubility in seawater increases as temperature decreases. The deepest water layers of the oceans were formed by the sinking of cold water in certain high-latitude regions (Chap. 8). Thus, the deepwater layers contain relatively high concentrations of dissolved carbon dioxide. In addition, deep waters below the warm surface water layer accumulate dissolved carbon dioxide as animals respire, and bacteria, and other decomposers convert organic matter to energy and carbon dioxide (Chap. 12). Therefore, older deep water (water that has been away from the surface longer) has a higher carbon dioxide concentration than newer deep water. The deep water of the Pacific Ocean is older than the deep water of the Atlantic Ocean (Chap. 8). Consequently, calcium carbonate is more soluble, and the CCD is shallower in the Pacific Ocean because of its higher dissolved carbon dioxide concentrations. The CCD is at approximately 4000 m in the Atlantic, at about 2500 m in the South Pacific, and at less than 1000 m in the North Pacific, which has the oldest deep waters with the highest carbon dioxide concentrations.
Anthropogenic Carbon Dioxide and the Carbonate Compensation Depth
The carbonate compensation depth (CCD) does not remain fixed but changes with climate, particularly with changes in the carbon dioxide concentrations of ocean water. An understanding of these changes is necessary to predict the fate of the excess carbon dioxide released to the atmosphere by the burning of fossil fuels (Chap. 7, CC9).
Atmospheric carbon dioxide, carbon dioxide dissolved in seawater (primarily as carbonate and bicarbonate ions), and carbon locked up in calcareous sediments or sedimentary rocks are all involved in the same biogeochemical cycle. Some of the excess carbon dioxide released to the atmosphere has been absorbed by ocean water, and some may have been captured in calcareous sediments. However, the higher carbon dioxide concentrations lower seawater pH and will move the CCD to shallower depth, causing more calcium carbonate to dissolve (Chap. 5). The effects of this ocean acidification on marine organisms in the surface layers of the oceans are a primary concern. However, as more calcium carbonate dissolves, this will result in an even higher concentration of carbonate and, therefore, carbon dioxide in the deep layers of the oceans. Eventually, this will cause more carbon dioxide to be released to the atmosphere when the deep waters are brought to the surface. How fast this will take place is, as yet, unknown. However, because atmospheric carbon dioxide and the CCD have varied substantially throughout the Earth’s history, we may be able to find an answer to this and other questions about the effects of ocean acidification in the record preserved in deep-sea sediments. Indeed, existing evidence suggests that release of carbon dioxide to the atmosphere from carbon dioxide-rich deep ocean waters may have caused or contributed to major warming events in Earth’s history, such as the interglacial periods of the last ice age.





