6.5: Hydrogenous Sediments
- Page ID
- 45545
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The input of hydrogenous sediments to the ocean floor is small in comparison with the inputs of biogenous and lithogenous material. Nevertheless, hydrogenous material can be an important component of the sediment, particularly in some areas where the accumulation rate of biogenous and lithogenous material is low.
Seawater is generally undersaturated with most dissolved substances. Consequently, hydrogenous sediments are formed under special conditions where the chemistry of ocean water is altered. Various types of hydrogenous sediment form under different sets of conditions. The types include hydrothermal minerals, manganese nodules, phosphorite nodules and crusts, carbonates, and evaporites.
Hydrothermal Minerals
Because the Earth’s crust is thin at the oceanic ridges, more heat flows through the seafloor from the mantle at the ridges than elsewhere. The excess heat drives a series of hydrothermal vents that discharge hot water (sometimes several hundred degrees Celsius) into the surrounding cold seawater. The hot water is anoxic, devoid of dissolved oxygen, when discharged, and it contains high concentrations of metal sulfides, including iron sulfide and manganese sulfide. These sulfides are soluble in the absence of dissolved oxygen, but they are oxidized to form insoluble hydrous oxides as the vent plumes mix with much larger volumes of the surrounding oxygenated seawater. The hydrous oxides precipitate to form a cloud of fine, metal-rich particles. These particles sink to the seafloor and accumulate as metal-rich sediment primarily in the area surrounding the vent.
In addition to iron and manganese, hydrothermal vent deposits contain high concentrations of other metals, including copper, cobalt, lead, nickel, silver, and zinc. Although some particles accumulate to form metal-rich sediment near the vents, many fine particles formed at the vents are probably transported large distances before settling and provide an input of hydrogenous particles to sediments throughout the deep-ocean basins. This may also be the origin of the material that forms the manganese nodules described later in this chapter.
The mechanism of hydrothermal vent circulation is not fully understood. The excess heat flow at the ridge is believed to drive the mechanism. Water within the rocks and sediments beneath the hydrothermal vents is heated and rises through the vents into the water above (CC1). This rising water is replaced by cold seawater that seeps through cracks in the rocks and sediment at other locations nearby and is transported to the vent through the rocks and sediment (Chap. 15). As the seawater migrates to the vent through the sediment and rocks, it is heated, its oxygen is depleted by reaction with sulfides, and its pH drops so that it leaches salts and metal sulfides from the sediment and surrounding rock before it is discharged at the vent. The vents are, in essence, parts of convection cells (CC3, Fig. 15-16).
Hydrothermal vents were not discovered until the late 1970s. They are surrounded by commercially attractive deposits of metal-rich sediment, and they also support unique communities of organisms, many of which are found nowhere else on the Earth (Chaps. 12, 15). Hydrothermal vents are present throughout the oceanic ridge system, but they are limited to small areas on the ridge that are often separated by substantial distances. Whether the metal-rich sediment is abundant enough to be commercially exploitable is not known. In addition, it is uncertain whether the unique biological communities could be safeguarded if the minerals were commercially exploited.
Along the axis of the Red Sea, where a new oceanic ridge is forming, hydrothermal vents have discharged huge quantities of hydrothermal minerals. The water in the deep basins of the Red Sea is isolated from and does not mix with open-ocean waters. As a result, hot water discharged by the hydrothermal vents has accumulated in the Red Sea deep basins because, even though the water is hot, it also has very high salinity and thus high density (CC6). The high density strongly inhibits vertical mixing of this deep water with Red Sea surface water. Therefore, the hot, high-salinity, deep water is also anoxic, which prevents the minerals discharged by the hydrothermal vents from being oxidized. These factors have caused metal-rich hydrothermal minerals to accumulate in large quantities in sediments, and high concentrations of suspended sediments to accumulate in Red Sea deepwater areas. The deposits are so extensive that test mining was performed in 1979, but no further mining has occurred yet, as the minerals are still available at lower cost from mining on land. Nevertheless, these hydrothermal minerals that have collected on the Red Sea floor may eventually represent an economic resource as mineral scarcities grow in the future.
Undersea Volcano Emissions
Undersea volcanic activity is not limited to the oceanic ridges. Volcanoes also erupt under the sea at some hot spots and at some convergent plate boundaries, such as the Mariana Arc. Sulfide-rich water, comparable to the hydrothermal fluids discharged at oceanic ridge vents, is discharged into the water column at some locations on these undersea volcanoes. The sediments in the areas surrounding these discharges sustain populations of biological communities similar to those found surrounding oceanic ridge hydrothermal vents, and sediments in some of these areas are likely to be similarly rich in some metals. There is particular interest in studying these communities and discharges because these undersea volcanoes are located at much shallower depths than most oceanic ridge hydrothermal vents, making them easier to reach for study. Even more importantly, some of these shallower vents are different because they discharge into the upper layers of the ocean, where photosynthesis also occurs. This raises many questions about how these relatively shallow discharges and communities may affect and/or interact with the chemistry and biology of the surrounding waters.
Manganese Nodules
Manganese nodules are dark brown, rounded lumps of rock, many of which are larger than a large potato. Enormous numbers of nodules litter parts of the deep-ocean floor (Fig. 6-12a). They are potentially valuable as a mineral resource because they usually consist of about 30% manganese dioxide, about 20% iron oxide, and up to 1–2% of other metals that are much more valuable, including copper, nickel, and cobalt.
Manganese nodules are probably formed by various mechanisms and from various sources of manganese, iron, and other elements. These chemicals may come from the dissolved ions in river water, from seawater leaching of volcanic materials in the ocean, or from hydrothermal vents on the oceanic ridges. At present, the major source of minerals in the nodules is thought to be oceanic ridge hydrothermal vents. Particles of colloidal size (submicroscopic) are hypothesized to form at the hydrothermal vents, be transported throughout the oceans, and accumulate by adsorption on the surface of the nodule, perhaps aided by microbial action.
Most nodules initially form around a large sediment particle (e.g., a shark’s tooth) and are then built up in layers (Fig. 6-12b) at the very slow rate of about 1 to 10 mm per million years. Nodules are most common where the sedimentation rate is extremely slow. In areas where sediment accumulates rapidly, nodules are buried by new sediment before they have time to grow. Where nodules do form, occasional disturbance of the nodule by marine organisms is hypothesized to be necessary to prevent the nodule from being buried.
The areas of greatest accumulation of manganese nodules are the deepest parts of the Pacific Ocean (Fig. 6-12c), where the sedimentation rate is very low. The greatest density of manganese nodules is in the region of the Pacific Ocean between about 10° and 20°N latitude (Fig. 6-12c).
Phosphorite Nodules and Crusts
Phosphorite is a mineral composed of up to 30% phosphorus. Phosphorite nodules form in limited areas of the continental shelf and continental slope and on some seamounts. Phosphorite nodule formation likely requires low dissolved oxygen concentrations in bottom waters and an abundant supply of phosphorus. These conditions are present in upwelling regions where productivity is high. In such regions, the decomposition of falling detritus depletes oxygen in the bottom waters and supplies relatively large quantities of phosphorus, which is released as phosphate when the organic matter decays.
Phosphorite nodules grow slowly (1 to 10 mm per 1000 years). In contrast to manganese nodules, they do not form concentric layers but instead grow only on the underside, accumulating phosphate released by the decomposition of organic matter. Because very large deposits of phosphates are available on land, phosphorite nodules are unlikely to become commercially valuable.
Carbonates
Many limestone rocks lack fossils. Some consist of biogenous sediments that have lost their fossils through diagenetic changes (discussed later in this chapter), but others consist of hydrogenous sediments formed by direct precipitation of calcium carbonate. Calcium carbonate precipitates from seawater under conditions that were apparently widespread in the oceans at various times in the past, but are now present only in very limited regions, such as the Bahamas.
Calcium carbonate precipitation occurs when the concentration in the water is higher than the calcium carbonate saturation solubility. This is more likely when water temperatures are high and the concentration of dissolved carbon dioxide is low, raising the pH (Chap. 5). High temperatures reduce the solubility of carbon dioxide in surface waters, allowing some dissolved carbon dioxide to escape into the atmosphere. Carbon dioxide concentrations in surface waters can also be reduced by high rates of photosynthetic production (Chap. 12, CC14).
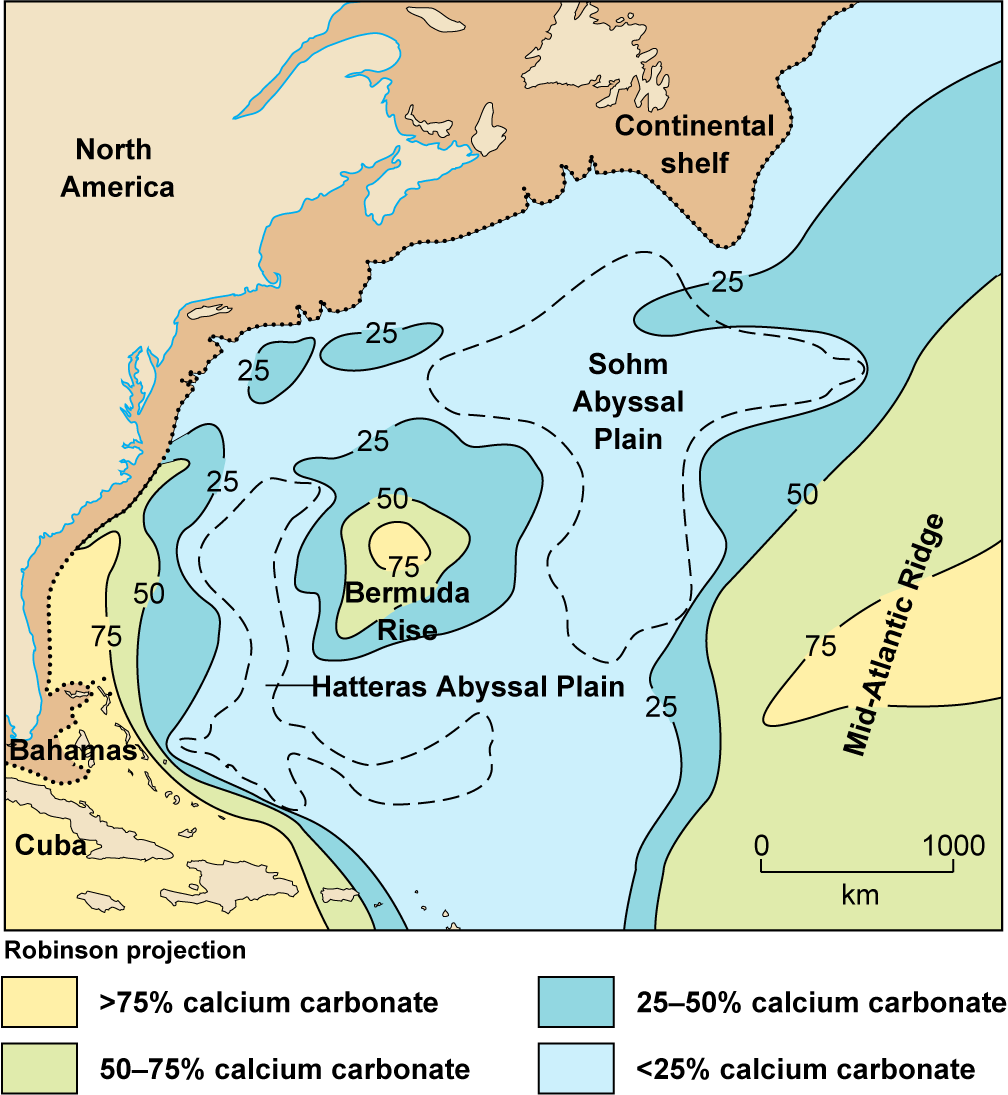
In the present-day oceans, both high temperature and high productivity appear to be necessary to reduce the calcium carbonate solubility enough to cause precipitation. For example, warm water flowing north through the Straits of Florida (Chap. 8) is upwelled across the shallow Bahama Banks (Fig. 6-13). Over the banks, it is heated further and sustains high primary productivity because it is nutrient-rich and sunlight is intense (Chap. 12, CC14). This causes the pH to rise, and as a result, calcium carbonate precipitates around suspended particles to form white, rounded grains called “ooids.” Ooids collect in shallow-water sediment in many areas. Where they are especially abundant in relation to other particles, the sediments are called “oolite sands.”
Evaporites
In marginal seas in arid climates, the evaporation rate of ocean water exceeds the rate of replacement by rainfall and rivers (Chap. 7). Under such conditions, salinity progressively increases until the seawater becomes saturated with salts. As evaporation continues, salts, called “evaporites,” are precipitated in succession: calcium and magnesium carbonates first, then calcium sulfate, and finally sodium chloride. Only a few marginal seas are evaporating in this way today, and even in these areas, salt precipitation occurs only on tidal flats or in shallow embayments around the perimeter of the sea. Areas where evaporites form today include the Dead Sea, Red Sea, and Persian Gulf. Such marginal seas must have been more abundant in the past because salt layers are present at many locations deep within ocean sediments, as well as in previously submerged parts of the continents.
Seismic profiles reveal several extensive layers of buried evaporite sediments, some more than 100 m thick, beneath the floor of the Mediterranean Sea. For such layers to have formed, the entire Mediterranean must have evaporated almost to dryness. This evaporation process apparently occurred several times when global sea level was lower and the connection between the Mediterranean and the Atlantic Ocean was broken at the sill that now lies underwater across the Straits of Gibraltar.


