5.1: Weathering, Erosion, and Soil
- Page ID
- 28238
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Weathering is a process that turns solid rock into smaller particles, called sediment or soil. The two types of weathering are mechanical (or physical) and chemical weathering, each of which will be discussed below, though their names give clues as to what they entail.
Erosion is a mechanical process, usually driven by water, wind, gravity, or ice, which transports sediment and soil from the place of weathering. Liquid water is the main agent of erosion. Erosion resistance is important in the creation of distinctive geological features. This is well-demonstrated in the cliffs of the Grand Canyon. The cliffs are made of rock left standing after less resistant materials have weathered and eroded away.
Mechanical Weathering
Mechanical weathering physically breaks bedrock into smaller pieces. The usual agents of mechanical weathering are pressure, temperature, freezing/thawing cycle of water, plant or animal activity, and salt evaporation.
Pressure Expansion

Bedrock buried deep within the Earth is under high pressure and temperature. When uplift and erosion brings bedrock to the surface, its temperature drops slowly, while its pressure drops immediately. The sudden pressure drop causes the rock to rapidly expand and crack; this is called pressure expansion. The "cracking" is the mechanical weathering.
Frost Wedging
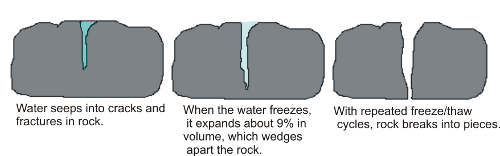
Frost wedging, also called ice wedging, uses the power of expanding ice to break apart rocks. Water works its way into various cracks, voids, and crevices. As the water freezes, it expands with great force, exploiting any weaknesses. When ice melts, the liquid water moves further into the widened spaces. Repeated cycles of freezing and melting eventually pry the rocks apart. The cycles can occur daily when fluctuations of temperature between day and night go from freezing to melting.
Root Wedging

Like frost wedging, root wedging happens when plant roots work themselves into cracks, prying the bedrock apart as they grow. Occasionally these roots may become fossilized. Tunneling organisms such as earthworms, termites, and ants are biological agents that induce weathering similar to root wedging.
Salt Expansion

Salt expansion, which works similarly to frost wedging, occurs in areas of high evaporation or near-marine environments. Evaporation causes salts to precipitate out of solution and grow and expand in the cracks in the rock, forcing them apart.
Chemical Weathering
Chemical weathering is the dominant weathering process in warm, humid environments. It happens when water, oxygen, and other reactants chemically degrade the mineral components of rock causing a change in composition. Higher temperatures accelerate chemical weathering rates.
Chemical and mechanical weathering work hand-in-hand via a fundamental concept called surface-area-to-volume ratio. Chemical weathering only occurs on rock surfaces because water and reactants cannot penetrate the solid rock. Mechanical weathering penetrates the bedrock, breaking large rocks into smaller pieces and creating new rock surfaces. This exposes more surface area to chemical weathering.
Carbonic Acid and Hydrolysis
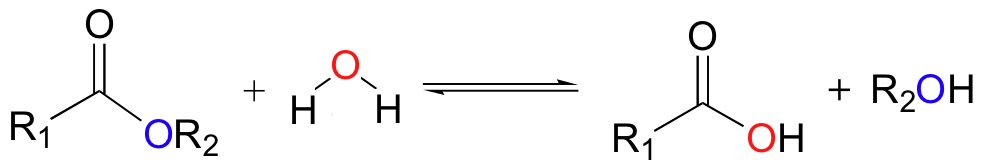
Carbonic acid (H2CO3) forms when carbon dioxide, the fifth-most abundant gas in the atmosphere, dissolves in water. This happens naturally in clouds, which is why precipitation is normally slightly acidic. Carbonic acid is an important agent in two chemical weathering reactions, hydrolysis, and dissolution.
Hydrolysis occurs via two types of reactions. In one reaction, water molecules ionize into positively charged H+ and OH− ions and replace mineral cations in the crystal lattice. In another type of hydrolysis, carbonic acid molecules react directly with minerals, especially those containing silicon and aluminum (i.e. Feldspars), to form molecules of clay minerals.
Hydrolysis is the main process that breaks down silicate rock and creates clay minerals. The following is a hydrolysis reaction that occurs when silica-rich feldspar encounters carbonic acid to produce water-soluble clay and other ions:
feldspar + carbonic acid (in water) → clay + metal cations (Fe++, Mg++, Ca++, Na+, etc.) + bicarbonate anions (HCO3-) + silica (SiO2)
Clay minerals are platy silicates or phyllosilicates (see Chapter 3, Minerals) similar to micas, and are the main components of very fine-grained sediment. The dissolved substances may later precipitate into chemical sedimentary rocks like evaporite and limestone, as well as amorphous silica or chert nodules.
Dissolution

Dissolution is a hydrolysis reaction that dissolves minerals in bedrock and leaves the ions in solution, usually in water. Some evaporites and carbonates, like salt and calcite, are more prone to this reaction; however, all minerals can be dissolved. Non-acidic water, having a neutral pH of 7, will dissolve any mineral, although it may happen very slowly. Water with higher levels of acid, natural or man-made, dissolves rocks at a higher rate. Liquid water is normally slightly acidic due to the presence of carbonic acid and free H+ ions. Natural rainwater can be highly acidic, with pH levels as low as 2 [3]. Regions with high humidity (airborne moisture) and precipitation experience more dissolution due to greater contact time between rocks and water.


Dissolution is also noteworthy for the special geological features it creates. In places with abundant carbonate bedrock, dissolution weathering can produce sinkholes or caves.
Timpanogos Cave National Monument in Northern Utah is a well-known dissolution feature. The figure shows a cave formation created from dissolution followed by precipitation—groundwater saturated with calcite seeped into the cavern, where evaporation caused the dissolved minerals to precipitate out.
Oxidation

Oxidation, the chemical reaction that causes rust in metallic iron, occurs geologically when iron atoms in a mineral bond with oxygen. Any minerals containing iron can be oxidized. The resultant iron oxides may permeate a rock if it is rich in iron minerals. Oxides may also form a coating that covers rocks and grains of sediment, or lines rock cavities and fractures. If the oxides are more susceptible to weathering than the original bedrock, they may create void spaces inside the rock mass or hollows on exposed surfaces.
The most commonly found mineral produced by iron-oxidation reactions is red or grey hematite. This iron oxide coat and bind mineral grains together into sedimentary rocks in a process called cementation and often give these rocks a dominant color. They color the rock layers of the Colorado Plateau, as well as Zion, Arches, and Grand Canyon National Parks.
Erosion

Erosion is a mechanical process, usually driven by water, gravity, (see Chapter 10), wind, or ice (see Chapter 14) that removes sediment from the place of weathering. Liquid water is the main agent of erosion.

Erosion resistance is important in the creation of distinctive geological features. This is well demonstrated in the cliffs of the Grand Canyon. The cliffs are made of rock left standing after less resistant materials have weathered and eroded away. Rocks with different levels of erosion resistant also create a unique-looking features called hoodoos in Bryce Canyon National Park and Goblin Valley State Park in Utah.
Soil
Soil is a combination of air, water, minerals, and organic matter that is made when weathering breaks down rock or sediment and creates weathering products in place. If the newly created material does not erode away, organisms can access the mineral content of the material. These organisms turn minerals, water, and atmospheric gases into organic substances that contribute to the soil.
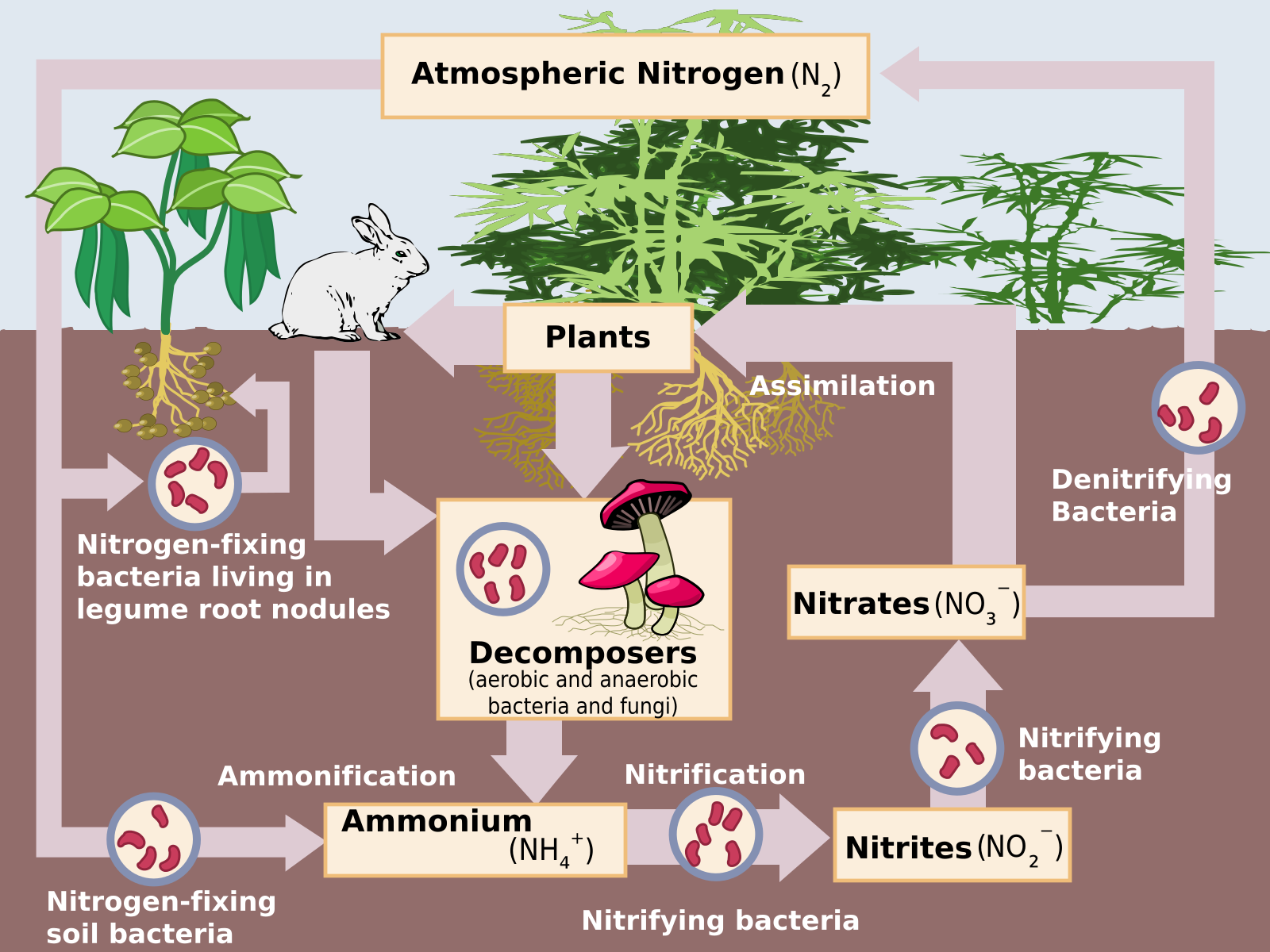

The nature of the soil, meaning its characteristics, is determined primarily by five components:
- The mineralogy of the parent material
- Relief or slope
- Amount of time that it has to form
- Climate
- The organisms that inhabit the soil.
For example, soil tends to erode more rapidly on steep slopes so soil layers in these areas may be thinner than in flood plains, where it tends to accumulate. The quantity and chemistry of organic matter of soil affect how much and what varieties of life it can sustain. Temperature and precipitation, two major weathering agents, are dependent on climate. Fungi and bacteria contribute organic matter and the ability of soil to sustain life, interacting with plant roots to exchange nitrogen and other nutrients [5].
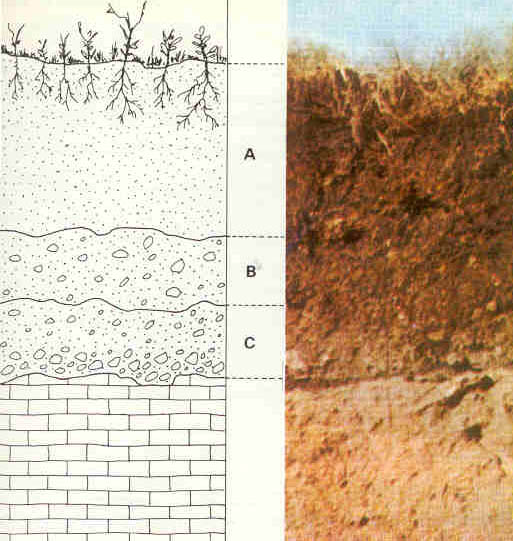
In well-formed soils, there is a discernable arrangement of distinct layers called soil horizons [6]. These soil horizons can be seen in road cuts that expose the layers at the edge of the cut. Each soil horizon reflects its organic material and mineral sediment composition. The horizons are assigned names and letters. The figure shows a simplified soil profile that uses commonly designated names and letters.
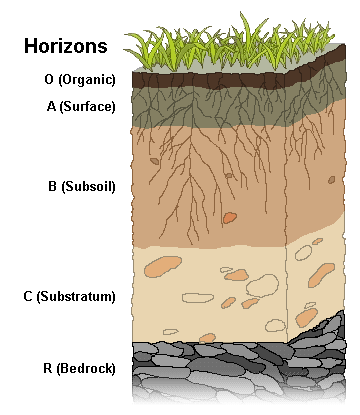
O Horizon: The top horizon is a thin layer of predominantly organic material, such as leaves, twigs, and other plant parts that are actively decaying into humus.
A Horizon: The next layer consists of humus (dark, organic material that forms in soil when plant and animal matter decays) mixed with mineral sediment. As precipitation soaks down through this layer, it leaches out soluble chemicals. In wet climates with heavy precipitation, this leaching out produces a separate layer called horizon E, the leaching or eluviation zone.
B Horizon: This layer consists of particles mixed with humus (dark, organic material that forms in soil when plant and animal matter decays); chemicals are removed from the upper layers and collect here. The subsoil is where mineral sediment is chemically weathered. The amount of organic material and the degree of weathering decrease with depth.
C Horizon: This is substratum and is a zone of weathering. Here, the parent material has just begun to weather. This layer contains no organic material.
R Horizon: The final layer consists of unweathered, parent material and fragments.

Not only is soil essential to terrestrial life in nature, but also human civilization via agriculture. Careless or uninformed human activity can seriously damage soil’s life-supporting properties. A prime example is the famous Dust Bowl disaster of the 1930s, which affected the midwestern United States. The damage occurred because of large-scale attempts to develop prairie land in southern Kansas, Colorado, western Texas, and Oklahoma into farmland [7]. Poor understanding of the region’s geology, ecology, and climate led to farming practices that ruined the soil profile.


