5.2: Soil Acidity and Adjusting Soil pH
- Page ID
- 14728
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Understand the origin of soil acidity.
- Measure soil pH with field and laboratory techniques.
- Determine the role of aluminum in soil acidity.
- State the relationship between cation exchange capacity, buffering capacity, and potential acidity.
- Write chemical reactions related to soil pH and liming.
- Know the objectives of liming and the factors affecting lime requirement.
- Measure limestone requirement.
- Determine limestone quality.
Managing soil pH is essential to creating ideal growth conditions for most plants. This is because the pH of the soil controls the solubility of nutrients as well as toxic metals. Because of this, most plants have a preferred range in soil pH. Under most cases, liming agents are added to soil to raise pH to the desired range. However, in some cases, lower soil pH is desired, which can be achieved using soil amendments such as elemental sulfur (S), or aluminum sulfate (more commonly referred to as “alum”). In either case, the pH tolerance of the target plant species, the properties of the soil, and the properties of the soil amendment must be considered to achieve the desired change in soil pH.
Materials
- Soils with low pH
- pH meter with a pH electrode
- pH test strips
- Beakers
- Glass stir rods
- Pure reagent grade calcium carbonate
- Pure reagent grade calcium oxide
- Pure reagent grade calcium sulfate (gypsum)
- Dolomitic limestone, coarse (sieved using a 20-40 mesh sieve)
- Dolomitic limestone, fine (sieved using a 100+ mesh sieve)
Recommended Reading & Viewing
- Soil pH Overview (CropWatch – Youth, 2013c)
- Soil pH Test (CropWatch – Youth, 2013d)
- Soil pH (USDA-NRCS, 2011)
- Soil pH (USDA-NRCS, 2014a)
- Liming Acid Soils (Whitney and Lamond, 1993)
Prelab Assignment
Using the recommended reading and the introduction to this lab, consider the questions listed below. These definitions/questions will provide a concise summary of the major concepts addressed in the lab. They are also useful as study notes for exams.
- Define pH and pOH. Show the formulas used to calculate both.
- Why is aluminum considered an acidic cation? Show the relevant reactions.
- List four negative consequences of low pH (acid) conditions in soil. List two negative consequences of high pH (basic) conditions in soil.
- Define buffering capacity. How does buffering capacity relate to cation exchange capacity?
- Define active acidity, salt-replaceable (exchangeable) acidity, and residual acidity.
- List various soil amendments that are used to increase the pH of an acid soil?
- Write a chemical reaction depicting how a typical liming material (calcitic limestone, burned lime, etc.) act to neutralize acidity.
- List various soil amendments used to acidify a soil and reduce the pH.
- What influences how much material is needed to increase the pH of an acid soil?
Introduction
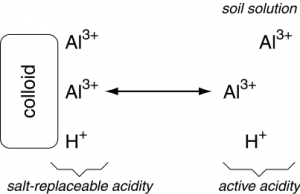
Soil acidity is largely controlled by the composition of ions on exchange sites on the colloidal fraction. The H+ cations are acidic by definition, and Al3+ cations are considered acidic because they react with H2O to produce Al(OH)3 and 3H+. The Ca2+, Mg2+, K+, Na+ cations, among others, are considered basic because they form strongly dissociated bases by reacting with OH–. These exchangeable cations on the exchange complex are in equilibrium with the cations in soil solution. Therefore, the nature of the exchangeable cations influences the composition of the soil solution.
The acidic cations adsorbed on the negative exchange sites are called the reserve (also residual or potential) and salt-replaceable (also exchangeable) acidity. The reserve and salt-replaceable acidity controls the level of soluble or active acidity in the soil solution. Only the active acidity is measured in a routine pH determination. The reserve and salt-replaceable acidity is always many times higher than the active acidity.
A soil is acid when hydrogen ions predominate in the soil. The degree of acidity is expressed in terms of pH, which is defined as the negative logarithm of the hydrogen ion activity. Therefore, the pH of a 0.01-molar hydrogen ion solution is
\[\text{pH}=-\text{log }\left(\dfrac{10^{-2}\text{ mol H}^+}{\text{L}}\right)=2\]
At pH 7, the concentration of H+ ions and OH- ions are equal, and the soil or solution is neutral. At pH values less than 7, the soil is acid; at values more than 7, the soil is alkaline. Most soils vary in pH from about 4 to 10. Soils in areas with high rainfall are generally acid with a pH less than 7. Soils developed in high-lime deposits often will be alkaline. Soils high in calcium seldom have pH values higher than 7.5, but the presence of large amounts of calcium carbonate may cause the pH to be as high as 8.5. Where the pH is higher than 8.5, an excess of sodium is highly probable.
The most desirable soil pH for most crops in Kansas is 6.8. However, crops like blueberries need a lower pH, and other crops, like alfalfa, need a higher pH. At soil pH less than 5.8, several problems may occur:
- Al and Mn toxicity
- Inhibited growth of N-fixing bacteria
- Possible deficiencies in Mg and/or Ca.
- P deficiency (P reacts with Fe and Al)
- At more than pH 7.5, other problems may occur:
- Deficiency of Fe, Mn, Cu, or Zn
- P deficiency (P reacts with Ca)
Buffering Capacity
Buffering capacity is a measure of the soil’s ability to resist a change in pH, directly related to the magnitude of the exchange capacity. Small fluctuations in acid or base content can occur without a noticeable pH change as cations are adsorbed or released from the exchange complex. Soils with the largest cation exchange capacity have the greatest buffering of a pH change. In other words, two soils may have the same pH (active acidity in soil solution), but the one with the largest cation exchange capacity will have the most acidity stored in reserve and therefore the highest buffering capacity or ability to resist a change in pH. For this reason, it takes less lime to increase the pH of a sandy soil (low CEC) by a given amount than it takes to increase the pH of a clay soil (higher CEC) the same amount.
Sources of Soil Acidity
Controlling soil pH is vital to optimal use and productivity of soils. Adding lime is the most effective and practical way to raise the pH of acid soils. Elemental sulfur, iron sulfate, or aluminum sulfate can be used to reduce soil pH. Because acidity is a concern in Kansas, we will focus on raising soil pH. Understanding the following equations should help you understand the sources of soil acidity and soil reactions to lime.
Acid cations adsorbed to colloids can be released to solution through cation exchange, in which cations such as Ca2+, Mg2+, K+, etc. can displace H+ and Al3+, forcing them into the soil solution, moving those acid cations from the salt-replaceable acidity pool to the active acidity pool. Notice that the reaction is reversible, so having high amounts of acid cations in solution could also cause the displacement of base cations from colloid exchange sites.
One product of respiration is CO2. In the soil, the respiration of bacteria, fungi, protists, roots, etc. contributes to a very high concentration of CO2 in the soil air. When CO2 becomes dissolved in the soil solution, it reacts with water to form carbonic acid – a weak acid that can release H+ into solution, thus lowering the soil pH. Note that this reaction is reversible.
\[\text{H}_2\text{O}+\text{CO}_2\leftrightarrow\text{H}_2\text{CO}_3\leftrightarrow\text{H}^++\text{HCO}_3^- \nonumber\]
Nitrification occurs under aerobic conditions. The oxidation of NH4+ to a final product of NO3– is facilitated by nitrosomonas and nitrobacter bacteria. The net reaction is shown below. Note that this reaction is not reversible, and that for every two moles of NH4+, there is four moles of H+ released.
\[2\text{NH}_4^++4\text{O}_2\rightarrow2\text{NO}_3^-+2\text{H}_2\text{O}+4\text{H}^+ \nonumber\]
Sulfur can be used as a soil amendment for lowering soil pH. A common example is for growing plants that prefer acidic conditions, such as blueberry, in soils that are neutral to alkaline. In this reaction, elemental sulfur is oxidized to form sulfuric acid – a strong acid. This is a microbial mediated process.
\[2\text{S}+3\text{O}_2+2\text{H}_2\text{O}\rightarrow\text{H}_2\text{SO}_4^+\leftrightarrow\text{SO}_4^{2-}+2\text{H}^+ \nonumber\]
Aluminum is considered an acidic cation due to the release of H+ during aluminum hydrolysis. For every one mole of Al3+, three moles of H+ are released into solution. Note that this reaction is reversible.
\[\text{Al}^{3+}+\text{H}_2\text{O}\leftrightarrow\text{Al(OH)}^{2+}+\text{H}^+ \nonumber\]
\[\text{Al(OH)}^{2+}+\text{H}_2\text{O}\leftrightarrow\text{Al(OH)}_2^{+}+\text{H}^+ \nonumber\]
\[\text{Al(OH)}_2^{+}+\text{H}_2\text{O}\leftrightarrow\text{Al(OH)}_3+\text{H}^+ \nonumber\]
Raising soil pH with lime
Standard agricultural lime, which is primarily calcium carbonate (CaCO3) is added to soils to increase soil pH. The CaCO3 reacts with water as shown below.
\[\text{CaCO}_3+\text{H}_2\text{O}\rightarrow\text{Ca}^{2+}+\text{HCO}_3^-+\text{OH}^- \nonumber\]
The Ca2+can displace other cations on the cation exchange, including H+ and Al3+ (salt-exchangeable acidity), thus releasing it into solution. However the Ca2+ does not react (neutralize) the acid cations. The acid cations are neutralized by HCO3– and OH–. An example of the overall chemical reaction following the addition of lime to acid soils is represented below.
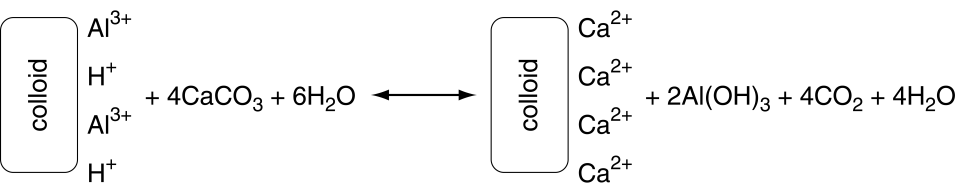
There are liming agents other than CaCO3. Because most of those other liming agents have different chemical formulas, the chemical reactions are different. This also means that the effectiveness of different liming agents vary from that of CaCO3.
Factors that Affect Liming Rates
The major factors that impact lime rates (the amount of lime required to raise the soil pH to a target pH) include the crop requirement; the type, size, and purity of the liming agent; the cation exchange capacity of the soil; and the pH of the soil.
Most plants have a range in soil pH in which they are most health, or produce the highest yield. This is because soil pH impacts nutrient availability. For example, some plants, like alfalfa and sweet clover, require more calcium than others, and thus require higher rates of liming. Others, like azaleas, cranberries, and blueberries, require more iron; which is more soluble at a lower pH. Therefore, it would take a larger application rate of lime to raise the soil to the desired soil pH for alfalfa than it would for blueberries.
It takes time for limestone to dissolve and replace hydrogen or aluminum on the soil exchange positions. Smaller lime particles have a larger surface area that is exposed and available to react, which reduces the time required for all of the lime to react. Therefore, finer liming agents are more effective at raising soil pH. Many limestones are predominately calcium carbonate (CaCO3), and some contain both CaCO3 and MgCO3. However most limestones contain some amount of impurities and inert material. Thus, a liming agent with lots of impurities (and less lime) is less effective at raising soil pH. Obviously, the purer the CaCO3, the more effective the lime is.
Soils with the same pH may require different amounts of limestone due to differences in CEC, which would imply differences in buffering capacities. For example, consider the amount of limestone necessary to raise the base saturation of two soils from 70% to 90% when one soil has a CEC of 15 cmolc/kg, and the other has a CEC of 40 cmolc/kg.
\[15\frac{\text{ cmol}_\text{c}}{\text{kg}}\times20\text{% increase}=3\frac{\text{ cmol}_\text{c}}{\text{kg}}\text{ basic cations required from lime} \nonumber\]
\[40\frac{\text{ cmol}_\text{c}}{\text{kg}}\times20\text{% increase}=8\frac{\text{ cmol}_\text{c}}{\text{kg}}\text{ basic cations required from lime} \nonumber\]
Lastly, soil pH is governed by base saturation. If other factors are constant, the lower the pH, the more lime that is required to achieve a desired pH. This is because at a low pH, a larger percentage of the CEC is occupied by acid cations, which requires larger amounts of lime to neutralize.
Activity 1: Determining pH With Indicator Strips (Field Method)
Of the several techniques available for determining pH, one that can be used easily in the field is the indicator strip method. This technique uses the principle of pH sensitivity of certain dyes, which cause differences in color across a range in pH. With the soils provided, complete the following pH determination:
Weigh 10.0 g of soil into a small plastic cup. Add 20 ml of distilled water and stir. Allow to stand for 5 minutes, occasionally stirring.
Using the pH indicator strips provided, dip the strip into the cup until the tip is wetted. Determine the pH by comparing the color change of the pH test strip to the color chart.
![]() Record the soil pH in Table 14.1.
Record the soil pH in Table 14.1.
Activity 2: Determining Soil pH with a pH Meter
Laboratory pH meters are more accurate than pH dyes and strips. The pH meter measures the hydrogen ion activity [H+] by measuring the electric potential across a thin, porous glass membrane at the base of the electrode. This potential changes in response to [H+], and by standardizing the instrument with buffers of known pH, we can measure the pH of any solution, including soil solutions.
Using the samples prepared in Activity 1, carefully place the electrode in the suspension. Gently swirl the electrode in the solution, and note the pH reading. Wait for the pH meter to reach a steady reading, indicated by the word “ready” on the screen.
![]() Record the value for this 1:2 soil-water suspension in Table 14.1.
Record the value for this 1:2 soil-water suspension in Table 14.1.
Activity 3: Determining a Need for Adding Limestone to the soil
To decide if a soil needs lime, you need to know only the pH, or the active acidity, of the soil solution. If the pH is less than or equal to 5.8, lime is generally recommended (the pH below which lime is required varies by region and intended crops).
![]() For each soil analyzed in Activity 2, decide if limestone is needed and record your decision in Table 14.1.
For each soil analyzed in Activity 2, decide if limestone is needed and record your decision in Table 14.1.
Table 14.1. Results for Activities 1-4: Determining Soil pH and Limestone Need
| Soil | Soil pH (strip) | Soil pH (meter) | Lime needed? | Lime Requirement |
|---|---|---|---|---|
| Yes/No | (lbs ECC/ac) | |||
| A | ||||
| B | ||||
| C | ||||
| D |
Activity 4: Determining How Much lime is needed
To decide how much lime a soil needs, you must determine the amount of reserve acidity in the soil. This reserve acidity is often called exchangeable acidity because it can be dissociated from the cation exchange complex through which it enters the soil solution. To determine the exchangeable acidity, a buffer solution of known pH is added to the soil. This buffer solution contains cations that will replace H+ and Al3+ on the exchange complex. The acidic cations removed from the exchange complex reduces the pH of the added buffer. Figure 14.1 illustrates the decrease in pH of a buffer solution as acid is added. Once a relationship is determined (shown by the slope of the line), you can add soil to the buffer solution (or buffer solution to the soil) to determine the exchangeable acidity.
In this exercise, we will use the SMP buffer – the buffer solution used by the K-State Soil Testing Lab. This buffer solution was designed to provide the liming rate when using the following formulas, depending on the region and target pH (see bullets).
\[\text{Target pH of }6.8= \nonumber\]
\[[25,620-(6,360\times\text{ buffer pH})+(391\times\text{buffer pH}\times\text{buffer pH})]\times\text{depth} \nonumber\]
- Depth is in inches
- Used for all crops in Southeast Kansas (east of Flint Hills and south of Highway 56)
- Used for alfalfa and clover in Northeast Kansas
- Lime is recommended if pH < 6.4
\[\text{Target pH of }6.0= \nonumber\]
\[[12,810-(3,180\times\text{ buffer pH})+(196\times\text{buffer pH}\times\text{buffer pH})]\times\text{depth} \nonumber\]
- Depth is in inches
- Used for all crops in Northeast Kansas other than alfalfa and clover
- Used for all crops in Central and Western Kansas
- Lime is recommended if pH < 5.8
\[\text{Target pH of }5.5= \nonumber\]
\[[6,405-(1,590\times\text{ buffer pH})+(98\times\text{buffer pH}\times\text{buffer pH})]\times\text{depth} \nonumber\]
- Depth is in inches
- Used if cash flow is limited or in lime availability problem areas in Central and Western Kansas
- Lime is recommended if pH < 5.5
This buffer contains chromium (Cr), a toxic heavy metal. Therefore, your lab instructor will perform the SMP buffer analysis. As a class, determine which soil-water mixtures from Activity 1 need lime (pH ≤ 6.4). To those solutions, add 10 ml of the SMP buffer solution, and stir with a glass rod. Allow the mixtures to stand for 30 minutes, which should be enough time for the acid cations to be displaced from the CEC and forced into solution. Read the pH on meter.
![]() Assuming the desired pH is 6.0, calculate the lime requirement, and record your results in Table 14.1.
Assuming the desired pH is 6.0, calculate the lime requirement, and record your results in Table 14.1.
Activity 5: Evaluating Liming Materials
The type of liming material and the size or fineness of the material determine how efficiently liming materials raise soil pH. This experiment was actually initiated earlier in the semester to allow time for the liming agents to react. Amending the soil with several different liming agents allows us assess the effects of particle size and liming material based on the relative changes in soil. The treatments included the following:
- Reagent grade CaCO3
- Reagent grade CaO
- Reagent grade CaSO4
- Coarse dolomitic limestone (35 mesh)
- Fine dolomitic limestone (120 mesh)
- Control (no amendments)
When this experiment was initiated, each lab section was divided into six groups, with each group responsible for one of the six treatments. Your laboratory instructor assigned a treatment to your group, and you completed the following steps:
- Label four plastic bags
- Weigh 20 g of air-dry soil into each plastic bag.
- Weigh 0.1 gram of designated liming material onto weighing paper.
- Add the liming material to the soil and mix thoroughly to distribute evenly in the soil.
- Add a few mL of water to each bag and mix.
- Close the bags to start incubation.
Now that the liming agents have had time to react, you will collect the results.
- Add 40 ml of distilled water to the plastic bag and mix. Let it sit for five minutes, mixing occasionally.
- Carefully place the electrode in the suspension, swirl the electrode, and determine the pH like you did previously in Activity 2.
- Record the pH for this 2:1 water-soil solution in Table 14.2 below.
Table 14.2. Liming material experiment results
| Treatment | Soil 1 (Sandy Soil) pH | Soil 2 (Clayey Soil) pH |
|---|---|---|
| Pure reagent grade CaCO3 | ||
| Pure reagent grade CaO | ||
| Pure reagent grade CaSO4 | ||
| Dolomitic limestone (35 mesh) | ||
| Dolomitic limestone (120 mesh) | ||
| Control (no amendments) |
![]() Record your group’s data on the table on the blackboard. Then record all of the class data onto the table above.
Record your group’s data on the table on the blackboard. Then record all of the class data onto the table above.
Activity 6: How Soil Characteristics affect Liming Reaction
To illustrate the effects of soil texture and organic matter on pH adjustment, your lab instructor added various rates of dolomitic limestone to a sandy soil, a clay soil, and an organic soil. The rates of limestone ranged from zero to 10 tons/acre. After several months, the following pH values were measured:
Table 14.3. Resulting pH values following lime additions
| Tons of added lime | Resulting pH Values | ||
|---|---|---|---|
| Sandy soil | Clay soil | Organic soil | |
| 0 | 4.5 | 4.5 | 3.5 |
| 1 | 5.5 | 4.9 | 3.6 |
| 2 | 6.5 | 5.2 | 3.7 |
| 3 | 7.0 | 5.5 | 3.8 |
| 4 | 7.2 | 5.7 | 3.9 |
| 5 | 7.3 | 5.9 | 4.0 |
| 6 | 7.4 | 6.1 | 4.2 |
| 7 | 7.5 | 6.3 | 4.5 |
| 8 | 7.6 | 6.5 | 5.0 |
| 9 | 7.7 | 6.7 | 5.5 |
| 10 | 7.7 | 7.0 | 6.0 |
![]() Plot the results in Table 14.3 into Figure 14.2 for all three soils, and connect the data points to form a line for each of the three soils. . Using the following graph to plot the results of pH against tons of lime added for each of the soils, answer the questions about this graph.
Plot the results in Table 14.3 into Figure 14.2 for all three soils, and connect the data points to form a line for each of the three soils. . Using the following graph to plot the results of pH against tons of lime added for each of the soils, answer the questions about this graph.
![]() What is the relationship between the amount of lime necessary to raise soil pH and the cation exchange capacity of the soil?
What is the relationship between the amount of lime necessary to raise soil pH and the cation exchange capacity of the soil?
![]() Should the pH goals for a clay soil and an organic soil always be the same? Explain your answer. HINT: Organic soils contain very little Al.
Should the pH goals for a clay soil and an organic soil always be the same? Explain your answer. HINT: Organic soils contain very little Al.
![]() If the sandy and the clayey soil were limed to achieve the same pH, which soil will probably need lime sooner? Why?
If the sandy and the clayey soil were limed to achieve the same pH, which soil will probably need lime sooner? Why?
Assignment: Online Quiz
A quiz for this lab will be available online. Please access it as directed by your instructor.


