14.7.3: Aragonite Group Minerals
- Page ID
- 18675
Aragonite CaCO3
Origin of Name
Named after the original locality in Aragon, Spain.



Hand Specimen Identification
Aragonite’s softness (H = 3.5 to 4), color, and association help identify it. Like calcite, it effervesces in cold dilute HCl. However, it does not have well-developed rhombohedral cleavage like calcite. If not well crystallized or showing orthorhombic cleavage, it may be difficult to distinguish from calcite. Aragonite, if white or clear, is also sometimes confused with strontianite.
Figure 14.381 shows a classic radiating cluster of orangish aragonite crystals (orthorhombic prisms) from Morocco. The color and morphology of this sample are diagnostic for aragonite. Figure 11.12 (Chapter 11) shows a specimen with crystals of similar morphology; the specimen is from Spain.
Figure 14.382 is a photo of white aragonite crystals with green malachite (a hydrated copper carbonate). When white, distinguishing aragonite from other white carbonates may be difficult. Figure 14.383 is a photo of helictites, a kind of cave formation made of aragonite; helictites form when CaCO3 precipitates from water.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 2.94 |
| cleavage/fracture | good (010), poor {110}/ subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless to white, and pale yellow or tan |
| streak | white |
Properties in Thin Section
Aragonite is similar to calcite in thin section but, because it is orthorhombic, displays parallel extinction. It is biaxial (-) with a small 2V. a = 1.530 , β = 1.681, γ = 1.685, δ = 0.155, 2V = 18°.
Crystallography
Aragonite is orthorhombic, a = 4.95, b = 7.96, c = 5.73, Z = 4; space group \(P\dfrac{2_1}{m}\dfrac{2_1}{c}\dfrac{2_1}{n}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Aragonite crystals are commonly acicular, tabular, prismatic, or fibrous. Crystals may form radiating sprays, crusts, or masses of many different morphologies. Contact and cyclic twins are common, sometimes giving it a pseudohexagonal appearance.
Structure and Composition
In aragonite, triangular (CO3)2- groups are in layers perpendicular to the c-axis, as in calcite. Alternate layers, however, have their (CO3)2- groups pointing in opposite directions. Ca is coordinated to nine oxygens in six surrounding (CO3)2- groups, giving orthorhombic (pseudohexagonal) symmetry. Solid solutions are much more restricted than for calcite. Aragonite is usually near end-member CaCO3, with only minor amounts of Sr, Pb, or Zn substituting for Ca.
Occurrence and Associations
Aragonite is found as disseminated carbonate in gypsum beds, as hot spring deposits, as precipitates from Ca-oversaturated waters, associated with sedimentary iron ores, in oxidized zones of ore deposits, in some cave formations, and in blueschist facies metamorphic rocks. It also occurs in shells and other organic carbonate material. Associated minerals typically include gypsum, siderite, celestite, sulfur, limonite, calcite, malachite, azurite, smithsonite, and cerussite.

Varieties
Flos ferri (Figure 14.384) is a coral-like form of aragonite associated with iron deposits.
Related Minerals
Aragonite has two significant polymorphs, calcite and vaterite. Strontianite, SrCO3; witherite, BaCO3; cerussite, PbCO3; and niter, KNO3, are all isostructural with aragonite.
Witherite BaCO3
Origin of Name
Named after D. W. Withering (1741–1799), who first demonstrated that witherite was different from barite.

Hand Specimen Identification
Clear or lightly colored translucent orthorhombic crystals, relatively high density (easily discerned by the heft test), effervescence with dilute HCl, and hardness of 3-3.5 identify witherite. It is occasionally confused with barite, but barite does not react with HCl. Figure 14.385 shows an example of witherite from Cumbria, England.
Physical Properties
| hardness | 3.5 |
| specific gravity | 4.29 |
| cleavage/fracture | good basal (010), poor {110} and {012}/uneven |
| luster/transparency | resinous/transparent to translucent |
| color | gray, white, or colorless |
| streak | white |
Properties in Thin Section
Witherite is similar to aragonite and other orthorhombic carbonates in thin section. It may be confused with strontianite, but the latter has lower RI and two good cleavages. Biaxial (-), α = 1.529 , β = 1.676, γ = 1.677, δ = 0.148, 2V = 16°.
Crystallography
Witherite is orthorhombic, a = 5.26, b = 8.85, c = 6.55, Z = 4; space group \(P\dfrac{2_1}{m}\dfrac{2_1}{c}\dfrac{2_1}{n}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Witherite crystals are orthorhombic, but multiple twinning yields pseudohexagonal pyramids. Columnar, globular, and botryoidal aggregates are common.
Structure and Composition
The structure of witherite is the same as that of aragonite. Minor Sr, Mg, and Ca may substitute for Ba.
Occurrence and Associations
Witherite is a rare low-temperature vein mineral, usually associated with galena and barite.
Related Minerals
Witherite has two high-temperature polymorphs. Strontianite, SrCO3; aragonite, CaCO3; cerussite, PbCO3; and niter, KNO3, are all isostructural with witherite.
Strontianite SrCO3
Origin of Name
Named after the first known locality at Strontian, Scotland.


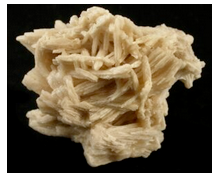
Hand Specimen Identification
Strontianite is generally colorless or lightly colored, vitreous, and translucent. The photos show two examples of this mineral. Strontianite forms orthorhombic crystals, has moderate density, and reacts with cold dilute HCl. When amorphous, and the orthorhombic nature of crystals cannot be seen, it is difficult to distinguish strontianite from other white minerals with moderate density and hardness. It may be confused with aragonite but has different cleavage.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 3.72 |
| cleavage/fracture | good prismatic {110}, poor (010)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | white, pale green, yellow, gray |
| streak | white |
Properties in Thin Section
Strontianite is similar to aragonite and other orthorhombic carbonates in thin section. Aragonite and witherite both have higher indices of refraction, aragonite has only one well-developed cleavage, and witherite has a larger 2V. Biaxial (-), a = 1.520 , β = 1.667, γ = 1.668, δ = 0.148, 2V = 7°.
Crystallography
Strontianite is orthorhombic, a = 5.13, b = 8.42, c = 6.09, Z = 4; space group \(P\dfrac{2_1}{m}\dfrac{2_1}{c}\dfrac{2_1}{n}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Strontianite is typically acicular but may be prismatic, fibrous, granular, or massive. Twins, creating a pseudohexagonal or lamellar appearance, are common.
Structure and Composition
Strontianite has one high-temperature polymorph. Aragonite, CaCO3; witherite, BaCO3; cerussite, PbCO3; and niter, KNO3, are all isostructural with strontianite. Substantial replacement of Sr by Ca or Ba is common; Pb may also be present.
Occurrence and Associations
Strontianite is an uncommon mineral. It occurs in hydrothermal veins with barite, celestite, and calcite. Hosts include limestones, sulfide veins, vugs, and concretions.
Related Minerals
Strontianite is one of the two important Sr-minerals; the other is celestite (Sr-sulfate).
Cerussite PbCO3
Origin of Name
From the Latin word cerussa, meaning “white lead.”

Hand Specimen Identification
High density (easily discerned by the heft test), color, and luster identify cerussite. It may be confused with anglesite (Pb-sulfate) but it has a different habit. Additionally, cerussite effervesces in cold dilute HCl; anglesite does not.
Figure 14.388 is a typical reticulated cluster of cerussite crystals. Cerussite is one of a small number of minerals that commonly display cyclic twins; Figure 14.389 shows an example from Namibia.
Physical Properties
| hardness | 3 – 3.5 |
| specific gravity | 6.5-6.6 |
| cleavage/fracture | good prismatic {110}, distinct {021}/conchoidal |
| luster/transparency | adamantine, vitreous, resinous, pearly, dull, earthy/translucent |
| color | colorless, white to light tan, less commonly gray, blue, or green |
| streak | white |
Properties in Thin Section
Cerussite is biaxial (-), a = 1.804 , β = 2.076, γ = 2.078, δ = 0.274, 2V = 9°.
Crystallography
Cerussite is orthorhombic, a = 5.15, b = 8.47, c = 6.11, Z = 4; space group \(P\dfrac{2_1}{m}\dfrac{2_1}{c}\dfrac{2_1}{n}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Cerussite crystals are variable but most commonly tabular. Twinning gives a pseudohexagonal appearance. Cerussite may also appear prismatic, acicular, granular, and massive. Coarse intergrowths and clusters with platy or reticulated fabric are typical.
Structure and Composition
Strontianite, SrCO3; aragonite, CaCO3; witherite, BaCO3; and niter, KNO3, are all isostructural with cerussite (see aragonite structure). It is generally quite close to end-member composition, although minor amounts of Ba, Sr, Ag, or Zn are sometimes present.
Occurrence and Associations
Cerussite is a common secondary lead mineral found in altered ore deposits. Typical associated minerals include galena, anglesite, limonite, and pyromorphite. It occurs in both veins and bedded deposits.
Related Minerals
Hydrocerussite, Pb3(CO3)2(OH)2, is a closely related mineral.


