14.1.3: Silicate Class - Chain Silicates
- Page ID
- 18646
14.1.3.1 Pyroxenes
Pyroxene Group Minerals
Orthopyroxene Series
enstatite Mg2Si2O6
ferrosilite Fe2Si2O6
Clinopyroxene (Diopside) Series
diopside CaMgSi2O6
hedenbergite CaFeSi2O6
Pyroxene Solid Solutions
hypersthene (Mg,Fe)2Si2O6
pigeonite (Ca,Mg,Fe)2Si2O6
augite (Ca,Mg,Fe,Na)(Mg,Fe,Al)(Si,Al)2O6
omphacite (Ca,Na)(Fe,Mg,Al)(Si,Al)2O6
aegirine Na(Al,Fe3+)Si2O6
Na- and Li-pyroxenes
jadeite NaAlSi2O6
spodumene LiAlSi2O6
All pyroxenes have a structure based on chains of SiO4 tetrahedra linked by shared (bridging) oxygen. Octahedral cations (Ca,Na,Mg,Fe,Al) occupy sites between nonbridging oxygens of adjacent chains. Small amounts of Al sometimes replace tetrahedral Si.
Mineralogists divide pyroxenes into two groups based on their crystal symmetry. Orthopyroxenes (orthorhombic) have two principal end members: enstatite, Mg2Si2O6, and ferrosilite, Fe2Si2O6. Compositions between are generally called hypersthene but more specific names can be found in the literature.
Clinopyroxenes (monoclinic) are of several types, and include diopside–hedenbergite solid solutions, high-temperature polymorphs of hypersthene, and a number of other Ca- and Na-bearing species. The most important end member is diopside, CaMgSi2O6. Many natural clinopyroxenes are close to diopside composition, so the name diopside is sometimes (imprecisely) used to refer to any green or greenish black clinopyroxene. Petrologists use the names hypersthene, pigeonite, augite, omphacite, and aegirine to describe solid-solution clinopyroxenes having specific physical properties and compositions.
Except at very high temperature, a large solvus exists between monoclinic calcic pyroxenes and orthorhombic Ca-free pyroxenes. Consequently, many mafic rocks contain both clinopyroxene and orthopyroxene.
For more general information about the pyroxene group, see Section 6.4.6 in Chapter 6.
Enstatite Mg2Si2O6
Origin of Name
From the Greek word enstates, meaning “adversary,” which refers to this mineral’s resistance to melting.


Hand Specimen Identification
Occurrence in mafic rocks, vitreous luster, green or dark color, and two cleavages at about 90° to each other identify pyroxene, but distinguishing enstatite from other pyroxenes can be problematic unless it has its distinctive olive-green color and orthorhombic shape. It may also be confused with an amphibole, but amphiboles have two cleavages at about 60° to each other.
Figure 14.102 shows a single crystal of enstatite from Mogok, Myanmar, that displays its orthorhombic character well. The massive, undistinctive, bronzite in Figure 14.103 is, however, more typical. Bronzite is a variety of enstatite that contains Fe.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 3.2 to 3.5 |
| cleavage/fracture | near 90o cleavage angle, two perfect prismatic {210}/uneven |
| luster/transparency | pearly, vitreous/translucent |
| color | olive-green or gray |
| streak | white |
Properties in Thin Section
Enstatite is nearly colorless in thin section. If Fe has replaced some of the Mg, it may exhibit slight to pronounced pink to green pleochroism. An 86° cleavage angle, parallel extinction in prismatic section, and relatively low birefringence distinguish it from clinopyroxene. Biaxial (+), α = 1.657 , β = 1.659, γ = 1.665, δ = 0.008, 2V = 54°.
Crystallography
Enstatite is orthorhombic, a = 18.22, b = 8.81, c = 5.21, Z = 4; space group \(P\dfrac{2_{1}}{b}\dfrac{2_{1}}{c}\dfrac{2_{1}}{a}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Enstatite is usually massive, but may be blocky, fibrous, or lamellar. Individual crystals may be prismatic or acicular.
Structure and Composition
Enstatite is one of two principal orthopyroxene end members; the other is ferrosilite, Fe2Si2O6. Enstatite and other pyroxenes contain chains of zigzagging SiO4 tetrahedra running parallel to the c-axis. Each tetrahedron shares two oxygens with neighbors in its chain and has one unshared oxygen at an apex pointing perpendicular to the c-axis. Pairs of chains face each other; Mg is located in four adjacent octahedral sites between unshared apices of tetrahedra.
Complete solid solution exists between enstatite and ferrosilite. Except at high temperature, only limited solid solution exists with calcic pyroxene. Mn, Cr, Al, and Ti may also be present in small amounts. See Section 6.4.6 in Chapter 6 for further discussion.
Occurrence and Associations
Enstatite is common in mafic igneous rocks, including gabbro, basalt, and norite, commonly associating with plagioclase and clinopyroxene. It is also found in some high-grade metamorphic rocks and is considered diagnostic for the granulite facies.
Varieties
Bronzite (Mg>>Fe) and hypersthene (Mg>Fe) are varietal names for Mg-Fe orthopyroxene.
Related Minerals
Enstatite is isostructural or isotypical with other pyroxenes. It is closely related to ferrosilite, Fe2Si2O6, and donpeacorite, (Mn,Mg)2Si2O6, two other pyroxenes. Most natural orthopyroxenes are predominantly enstatite–ferrosilite solid solutions with enstatite as their major component.
Diopside CaMgSi2O6
Origin of Name
From the Greek words dis and opsis, meaning “two” and “appearance,” referring to the fact that diopside appears different when viewed in different ways.


Hand Specimen Identification
An olive-green color and occurrence in marbles help identify diopside, although it may sometimes be confused with olivine. Diopside also occurs in association with other mafic minerals in mafic rocks, but in such occurrences, its color is more variable. Pyroxene cleavage – two cleavages at 86° to each other – aid identification.
Figure 14.104 shows green diopside crystals in a marble from the Adirondack Mountains. Figure 4.23 is a closeup view of diopside in Adirondack marble. The white mineral is calcite. Figure 13.35 is a photo of euhedral diopside, also from the Adirondacks. Figure 14.105 is a photo of green euhedral diopside with red garnet and darker-colored clinochlore.
Diopside may be confused with other pyroxenes or with hornblende, but the latter has two cleavages at 60° to each other. Diopside may contain substantial Fe, Al, or other impurities; exact composition cannot be determined without analytical data. Diopside is often white to green or pale green, especially in marbles; augite tends to be darker green or black.
Physical Properties
| hardness | 5.5 to 6.5 |
| specific gravity | 3.2 to 3.5 |
| cleavage/fracture | near 90o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | light green, variable |
| streak | white |
Properties in Thin Section
Diopside is colorless in thin section when Fe-free. With increasing iron content, it may become pleochroic in greens or browns. Higher birefringence and inclined extinction distinguish diopside and other clinopyroxenes from orthopyroxene. Grains are prismatic or blocky, depending on orientation. Extinction angle, optic sign, and 2V help distinguish the different clinopyroxenes, but telling them apart may be difficult. Biaxial (+), α = 1.665 , β = 1.672, γ = 1.695, δ = 0.030, 2V = 56° to 62°.
Crystallography
Diopside is monoclinic, a = 9.7, b = 8.9, c = 5.25, β = 105.83°, Z = 4; space group \(C\dfrac{2}{c}\); point group \(\dfrac{2}{m}\).
Habit
Prismatic crystals often have a square or octahedral cross section. Diopside may also be massive or finely disseminated. Polysynthetic twins are common but are generally not be visible without a microscope.
Structure and Composition
The structure of diopside is similar to that of other pyroxenes. Chains of SiO4 tetrahedra run parallel to the c-axis, with octahedral Ca and Mg connecting opposing chains to each other. Ca and Mg occupy different structural sites; Ca:Mg ratios are always ≤1. Complete solid solution is possible between diopside (CaMgSi2O6), hedenbergite (CaFeSi2O6), and johannsenite (CaMnSi2O6). Small amounts of Al, Na, Ti, and Cr may be present.
Occurrence and Associations
Diopside is a common pyroxene. Diopside-rich clinopyroxene, generally called augite, is found in mafic and ultramafic igneous rocks, associated with plagioclase, hornblende, and olivine. Near end-member diopside is found in marbles where it is associated with calcite, quartz or forsterite, tremolite, scapolite, and garnet. It is also found in medium- and high-grade metamorphosed mafic rocks.

Varieties
Chrome diopside is a chromium-rich variety known for its vivid green color (Figure 14.106).
Related Minerals
All pyroxenes are closely related. Hedenbergite, CaFeSi2O6, is the iron end member of the diopside series. Augite, (Ca,Mg,Fe,Na)(Mg,Fe,Al)Si2O6, is a related pyroxene with slightly different structure. Pigeonite is a high-temperature, subcalcic pyroxene with compositions that approach those of Fe-Mg diopside.
Pigeonite (Ca,Mg,Fe)2Si2O6
Origin of Name
Named after the place where this mineral was originally found, Pigeon Cove, Minnesota.

Hand Specimen Identification
Occurrence as phenocrysts in a volcanic rock, form, two cleavages at 86°, and color may identify pigeonite, but this mineral occurs in other rocks, and distinguishing pigeonite from the other dark-colored pyroxenes is difficult. Only X-ray studies can make the distinction. Figure 14.107 shows a basalt from the moon that contains black crystals of pigeonite.
Physical Properties
| hardness | 6 |
| specific gravity | 3.4 |
| cleavage/fracture | near 90o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | pearly, vitreous/sometimes translucent |
| color | brown, green, black |
| streak | white |
Properties in Thin Section
Pigeonite is similar to other clinopyroxenes in thin section (see the optical properties of diopside) but has a lower 2V. Biaxial (+), a = 1.69 , β = 1.69, γ = 1.72, δ = 0.025, 2V = 0° to 32°.
Crystallography
Pigeonite is monoclinic, a = 9.73, b = 8.95, c = 5.26, β = 108.55°, Z = 4; space group \(C\dfrac{2}{c}\); point group \(\dfrac{2}{m}\).
Habit
Rare euhedral crystals are prismatic. Pigeonite is most common as subhedral or anhedral grains in volcanic rock.
Structure and Composition
Pigeonite is similar in structure to other pyroxenes such as enstatite and diopside, and may contain the same impurities. It contains more Ca than enstatite and less than diopside, resulting in a slightly different structure.
Occurrence and Associations
Pigeonite is only found in high-temperature igneous rocks that have cooled rapidly. If it forms in plutonic rocks, it generally inverts to lower-temperature pyroxenes with cooling. Its former presence, however, may be inferred from textural features or from the presence of exsolved grains containing augite and orthopyroxene.
Related Minerals
Pigeonite is closely related to other pyroxenes, especially augite.
Augite (Ca,Mg,Fe,Na)(Mg,Fe,Al)(Si,Al)2O6
Origin of Name
From the Greek word augites, meaning “brightness,” referring to its shiny cleavage surfaces.


Hand Specimen Identification
Occurrence in mafic igneous rocks, form, two cleavages at 86°, and color identify pyroxene, but differentiating augite from other dark-colored pyroxenes is problematic without X-ray data. White to green or pale green pyroxenes are often diopside; augite tends to be dark green or black. Augite is occasionally confused with hornblende, but augite’s near 90o cleavage angle contrasts with hornblende’s 60o cleavage angle.
The two photos seen above show some classic examples of euhedral augite. Most augite, however, comprises small anhedral grains in plutonic or volcanic rocks. Figure 13.36 (from Chapter 13) shows an uncommon occurrence – a cluster of black augite crystals.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 3.2 to 3.4 |
| cleavage/fracture | near 90o cleavage angle, two perfect {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | black, dark green |
| streak | white |
Properties in Thin Section
Augite is similar to other clinopyroxenes in thin section. Grains are prismatic or blocky, depending on orientation. Color may be various shades of light brown, yellow-brown, or green. Augite may exhibit weak pleochroism. Extinction angle, optic sign, and 2V help distinguish the different clinopyroxenes, but telling them apart may be difficult. Biaxial (+), a = 1.671 to 1.735 , β = 1.672 to 1.741, γ = 1.703 to 1.761, δ = 0.030, 2V = 25° to 60°.
Crystallography
Augite is monoclinic, a = 9.8, b = 9.0, c = 5.25, β = 105°, Z = 4; space group \(C\dfrac{2}{c}\); point group \(\dfrac{2}{m}\).
Habit
Individual augite crystals are typically poorly formed stubby black or green prisms with octagonal or square cross sections. Simple and polysynthetic twins may be present. Large euhedral crystals, like those seen in the two figures above, are relatively rare.
Structure and Composition
The structure of augite is similar to that of the other clinopyroxenes (see diopside structure). Its chemistry is, however, more complex and variable. Ca:Mg:Fe ratios vary. Significant solid solution occurs with jadeite (NaAlSi2O6;), aegirine (NaFeSi2O6), and Ca-Tschermak’s pyroxene (CaAl2SiO6). Ti, Li, Mn, and a number of other elements may also be present in small amounts.
Occurrence and Associations
Augite is the most common pyroxene found in mafic to intermediate igneous rocks, both plutonic and volcanic. Associated minerals include hornblende and plagioclase.

Related Minerals
Augite is equivalent in composition to diopside with many impurities. It is structurally and chemically closely related to other pyroxenes, especially pigeonite, and to pyroxenoids. Omphacite is a bright green variety of augite rich in Na and Al. Figure 14.110 show green omphacite in an eclogite from Norway. The rock also contains garnet, quartz, and a small amount of kyanite (near the center of the specimen).
Jadeite NaAlSi2O6
Origin of Name
Name of unknown origin. The term jade refers to either jadeite or to the amphibole, nephrite.
Hand Specimen Identification
Association with other high-pressure minerals, form, two (rarely seen) cleavages at near 90°, green color, and tenacity identify jadeite. It is distinguished from nephrite (a green amphibole) by its luster. An optical microscope may be needed to confirm identification.
The photos below show four examples of jadeite. Sometimes this mineral has a vivid green hue, but more subdued green colors, such as the color in Figure 14.113, are common. Jadeite has historically been used to make jewelry, in art projects, or as ornamental stone. Figure 14.114 shows examples of a figurine and cabochon made from jadeite.




Physical Properties
| hardness | 6.5 to 7 |
| specific gravity | 3.30 |
| cleavage/fracture | 86o cleavage angle, two perfect {110}/uneven |
| luster/transparency | vitreous, greasy/translucent |
| color | variable shades of green to white; may be emerald green |
| streak | white |
Properties in Thin Section
Jadeite is colorless to very pale green in thin section. Birefringence is low; anomalous blue interference colors are common, maximum interference colors are first-order red or yellow. Jadeite exhibits typical clinopyroxene shape and cleavage but has a higher 2V than most. Biaxial (+), α = 1.65 , β = 1.66, γ = 1.67, δ = 0.02, 2V = 70° to 75°.
Crystallography
Jadeite is monoclinic, a = 9.50, b = 8.61, c = 5.24, β = 110.46°, Z = 4; space group \(C\dfrac{2}{c}\); point group \(\dfrac{2}{m}\).
Habit
Jadeite is usually granular, forming tenacious masses; less commonly, it is in prismatic or tabular crystals.
Structure and Composition
Jadeite is similar in structure to other clinopyroxenes and may contain some of the same impurities (see the composition of diopside). It forms limited solid solutions with aegirine, NaFeSi2O6, with omphacite, (Ca,Na)(Fe,Mg,Al)Si2O6, and with other pyroxene end members.
Occurrence and Associations
Jadeite is a high-pressure pyroxene found in metamorphic rocks of the blueschist facies. It is typically associated with other high-pressure minerals such as glaucophane, lawsonite, or aragonite, and with quartz and epidote. Omphacite, Na-rich augite that occurs in eclogites, is a solid-solution pyroxene that may be mostly jadeite.
Related Minerals
Jadeite is closely related to other Na-pyroxenes: aegirine, NaFeSi2O6; omphacite, (Ca,Na)(Fe,Mg,Al)Si2O6; and aegirine-augite, (Ca,Na)(Fe2+,Fe3+,Mg)Si2O6. It is chemically similar to nepheline, (Na,K)AlSiO4, and to albite, NaAlSi3O8.
Spodumene LiAlSi2O6
Origin of Name
From the Greek word spodoumenos, meaning “ashes.”


Hand Specimen Identification
Spodumene is typically found in pegmatites where it may form very large crystals such as the crystal seen in Figure 14.115. Prismatic cleavage, near 90o cleavage angle, hardness, light whitish color, and association help identify it, but it may be difficult to tell from feldspars or scapolite. This mineral commonly breaks into splintery cleavage fragments that have the appearance of a log or of petrified wood (Figure 14.116). Less commonly, spodumene occurs as clear gem crystals; Figures 14.117 and 14.118 below show two examples.
Physical Properties
| hardness | 6.5 to 7 |
| specific gravity | 3.15 |
| cleavage/fracture | near 90o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | typically light-colored white, gray, or colorless, less commonly, pink, green, yellow, purple, or tan |
| streak | white |
Properties in Thin Section
Spodumene is similar to other clinopyroxenes in thin section. Its occurrence in pegmatites and its small extinction angle (20° to 26°) help identify it. Biaxial (+), α = 1.65 , β = 1.66, γ = 1.67, δ = 0.02, 2V = 60° to 80°.
Crystallography
Spodumene is monoclinic, a = 9.52, b = 8.32, c = 5.25, β = 110.46°, Z = 4; space group \(C\dfrac{2}{c}\); point group \(\dfrac{2}{m}\).
Habit
Prismatic crystals with well-developed {100} faces showing vertical striations are common for spodumene. Crystals are often polysynthetically twinned and may be very large. In some pegmatites they are tens of meters long. Spodumene also occurs as cleavable masses.
Structure and Composition
Spodumene is a pyroxene with structure similar to that of diopside. Minor Na substitutes for Li, Fe, Ca, Mn, Mg, and rare earths are also present in small amounts.
Occurrence and Associations
Spodumene is found in granitic pegmatites, where it associates with K-feldspar, muscovite, quartz, tourmaline, beryl, and lepidolite.


Varieties
Hiddenite is a name given to emerald-green spodumene; kunzite to lilac/pink spodumene; and triphane to colorless or yellow spodumene. The photos in these two figures show gemmy examples of triphane and kunzite from Afghanistan.
Related Minerals
Spodumene shares many properties with other pyroxenes. It is similar in composition to eucryptite, LiAlSiO4.
1.3.2 Amphiboles
Amphibole Group Minerals
Monoclinic Amphiboles: Cummingtonite Series
cummingtonite (Mg,Fe)7Si8O22(OH)2
grunerite Fe7Si8O22(OH)2
Monoclinic Amphiboles: Actinolite Series
tremolite Ca2Mg5Si8O22(OH)2
actinolite Ca2(Mg,Fe)5Si8O22(OH)2
Hornblende
hornblende (K,Na)0-1(Ca,Na,Fe,Mg2)2(Mg,Fe,Al)5(Si,Al)8O22(OH)2
Na-amphiboles
glaucophane Na2Mg3Al2Si8O22(OH)2
riebeckite Na2(Fe,Mg)3(Fe,Al)2Si8O22(OH)2
Orthorhombic Amphiboles
anthophyllite (Mg,Fe)7Si8O22(OH)2
gedrite (Mg,Fe,Al)7(Si,Al)8O22(OH)2
Amphiboles are double-chain silicates. They share many physical and chemical properties with pyroxenes. Major chemical variations mirror those of the pyroxene group.
Like the pyroxenes, amphiboles may be either monoclinic or orthorhombic. Calcic amphiboles are monoclinic; Ca-free amphiboles are generally orthorhombic. A solvus between calcic and noncalcic amphiboles results in many rocks containing two or, in some cases, three different amphibole species.
Many end-member amphiboles have specific names, the most important are listed in the box here (see hornblende entry, below, for additional names). The detailed descriptions that follow do not include gedrite and riebeckite because they are similar to the more common anthophyllite and glaucophane.
Petrologists use the name hornblende to refer to the common black amphibole found in many igneous and metamorphic rocks. Hornblendes have complex and highly variable chemistry; the formula in the box above only partially reflects the variations.
For more general information about the amphibole group, see Section 6.4.6 in Chapter 6.
Cummingtonite (Mg,Fe)7Si8O22(OH)2
Origin of Name
Named after Cummington, Massachusetts, its type locality.


Hand Specimen Identification
Prismatic bladed or acicular habit, two perfect cleavages intersecting at near 60° when viewed in basal section, color (if light brown or gray), and association identify cummingtonite. It may be confused with other amphiboles, especially anthophyllite and gedrite.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 2.9 to 3.2 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous, silky, fibrous/ transparent to translucent |
| color | light brown, gray, whitish, or green |
| streak | white |
Properties in Thin Section
Cummingtonite is colorless to pale green in thin section and exhibits weak pleochroism. Interference colors may be up to second order. Basal sections show typical amphibole cleavage displaying a 56° cleavage angle. Extinction is inclined, polysynthetic twinning is common, and birefringence is greater than for anthophyllite–gedrite. Biaxial (+), α = 1.644 , β = 1.657, γ = 1.674, δ = 0.030, 2V = 80° to 90°.
Crystallography
Cummingtonite is monoclinic, a = 9.51, b = 18.19, c = 5.33, β = 101.83°, Z = 2; space group \(C\dfrac{2}{m}\); point group \(\dfrac{2}{m}\).
Habit
Cummingtonite forms prismatic, fibrous crystals; aggregates of radiating fibers or blades are common. See Figures 14.119 and 14.120, above.
Structure and Composition
Cummingtonite, like other amphiboles, has a double-chain structure. SiO4 tetrahedra are linked to make double chains that run parallel to the c-axis. Each tetrahedron shares two or three oxygen with neighbors, and has an unshared oxygen at the vertex pointing perpendicular to c. Chains are paired; unshared oxygens point toward each other and are bonded to the five octahedral cations occupying sites between them. A complete solid solution series exists between Mg-cummingtonite, Mg7Si8O22(OH)2, and grunerite, Fe7Si8O22(OH)2. The name cummingtonite is given to intermediate compositions, (Mg,Fe)7Si8O22(OH)2, with Mg > Fe. Substantial Mn may replace Mg; Al and Ca may be present in small amounts.
Occurrence and Associations
Cummingtonite occurs in mafic or marly medium-grade metamorphic rocks. Common associated minerals include other amphiboles (hornblende, actinolite, or anthophyllite), garnet, plagioclase, and cordierite. Cummingtonite also occurs in a few rare kinds of igneous rocks.
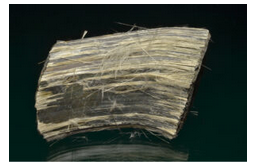
Varieties
Amosite is an asbestiform amphibole similar to Fe-rich cummingtonite. Figure 14.121 shows an example.
Related Minerals
Cummingtonite is closely related to the other amphiboles and is polymorphic with members of the anthophyllite series.
Grunerite Fe7Si8O22(OH)2
Origin of Name
Named after L. E. Grüner, a nineteenth-century mineralogist who first analyzed grunerite.


Hand Specimen Identification
Green color, acicular or bladed crystals, radiating habit, and association with other Fe-rich minerals help identify grunerite. It can, however, be difficult to distinguish from other amphiboles, especially cummingtonite. If crystals are large enough, they show two prominent cleavages at 56o to each other, confirming amphibole identity but not the species.
Commonly, grunerite crystals are in the form of fine needles or blades, as in the samples of Figures 14.122 adn 14.123. Needles may be aligned with either parallel or radiating textures, but radiating is more diagnostic.
Physical Properties
| hardness | 6 |
| specific gravity | 3.1 to 3.6 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | dark green or brown |
| streak | white |
Properties in Thin Section
Grunerite is similar to other members of the cummingtonite–grunerite series (see cummingtonite optics), but exhibits less pleochroism than cummingtonite, has an extinction angle of 10° to 15° prismatic cleavage, and may show interference colors up to third order. Biaxial (-), α = 1.69 , β = 1.71, γ = 1.73, δ = 0.040, 2V = 80° to 90°.
Crystallography
Grunerite is monoclinic, a = 9.6, b = 18.3, c = 5.3, β = 101.8°, Z = 2; space group \(C\dfrac{2}{m}\); point group \(\dfrac{2}{m}\).
Habit
Grunerite typically forms fibrous, bladed, or columnar crystals, often radiating.
Structure and Composition
Grunerite is an end member of the cummingtonite–grunerite series. Structure and composition are analogous to cummingtonite. The name grunerite is, by definition, restricted to compositions close to end-member Fe7Si8O22(OH)2.
Occurrence and Associations
Grunerite is found with Fe-rich minerals such as magnetite, hematite, minnesotaite, hedenbergite, fayalite, or garnet in metamorphosed Fe-rich sediments.
Related Minerals
Grunerite is closely related to the other amphiboles, especially cummingtonite.
Tremolite Ca2Mg5Si8O22(OH)2
Origin of Name
Named after Val Tremola, Switzerland, where it was first found.



Hand Specimen Identification
Occurrence in metacarbonates, perfect prismatic cleavages, 56° cleavage angle when viewed in basal section, fibrous/bladed or thin columnar crystals, and generally very light color identify tremolite. Cleavage angle distinguishes it from pyroxenes and pyroxenoids; light color distinguishes it from hornblende. It may sometimes be confused with vesuvianite or wollastonite.
The fragment of tremolite in Figure 14.124 shows amphibole cleavage well; otherwise the specimen is quite nondescript. This is typical for tremolite. When euhedral, tremolite commonly forms white blades, sometimes in masses as seen in the photo in Figure 14.125. Figure 14.126 shows an uncommon occurrence – a transparent single needle of tremolite.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 3.0 to 3.3 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | typically white, less commonly light green |
| streak | white |
Properties in Thin Section
Generally colorless when Fe-free, tremolite may be green and pleochroic when Fe is present. Amphibole cleavage angles (56° and 124°), 10° to 21° extinction angle in prismatic section, large 2V, and upper first- to second-order interference colors identify tremolite. Biaxial (-), α = 1.608 , β = 1.618, γ = 1.630, δ = 0.022, 2V = 85°.
Crystallography
Tremolite is monoclinic, a = 9.86, b = 18.11, c = 5.34, β = 105.00°, Z = 2; space group \(C\dfrac{2}{m}\); point group \(\dfrac{2}{m}\).
Habit
Tremolite is typically prismatic. It may be in radiating or parallel blades, fibrous, asbestiform, or columnar. It is commonly twinned parallel to {100}.
Structure and Composition
Tremolite, Ca2Mg5Si8O22(OH)2, is the Mg end member of the calcic amphibole series. Complete solid solution exists between tremolite and Fe-actinolite, Ca2Fe5Si8O22(OH)2. Intermediate compositions are simply termed actinolite. Like other amphiboles, tremolite has a double-chain structure. SiO4 tetrahedra are linked to make double chains that run parallel to the c-axis. Each tetrahedron shares two or three oxygen with neighbors and has an unshared oxygen at the vertex pointing perpendicular to c. Chains are paired; unshared oxygens point toward each other and are bonded to the five octahedral cations occupying sites between them. The two larger octahedral sites are occupied by Ca. Other alkalis and alkaline earths may substitute in small amounts for Ca, and some Al may be present in either the octahedral or tetrahedral sites. If impurities are present in sufficient quantities, the amphibole becomes dark and, in the absence of analytical data, we call it hornblende.
Occurrence and Associations
Tremolite is one of the first minerals to form when impure carbonates are metamorphosed. It is associated with calcite, dolomite, talc, quartz or forsterite, diopside, and phlogopite.

Varieties
Hexagonite is a red to pink, lilac or purple Mn-rich variety of tremolite (Figure 14.127).
Related Minerals
All amphiboles are structurally similar. Tremolite is closely related to Fe-actinolite Ca2Fe5Si8O22(OH)2, the other principal calcic amphibole end member.
Actinolite Ca2(Fe,Mg)5Si8O22(OH)2
Origin of Name
From the Greek word actis (ray), referring to its common habit of radiating needles.


Hand Specimen Identification
A bladed, columnar, or needle-like habit, forming in masses, prismatic cleavages, 56° and 124° cleavage angles, and distinctive green color usually serve to identify actinolite. It is sometimes confused with epidote because of its green color. Mg:Fe ratios of actinolite may vary; exact composition cannot be determined in hand specimen.
The photo in Figure 14.128 shows a mass of classic bladed green actinolite. Most actinolite specimens have an appearance quite similar to this one. The photo in Figure 14.129 shows a rare occurrence – a spectacular cluster of parallel euhedral actinolite crystals from Namibia.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 3.0 to 3.3 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | dark green |
| streak | white |
Properties in Thin Section
Actinolite is similar to tremolite in thin section (see tremolite), but is generally more strongly colored and pleochroic. Biaxial (-), α = 1.66 to 1.67, β = 1.62 to 1.68, γ = 1.63 to 1.69, δ = 0.03, 2V = 70° to 80°.
Crystallography
Actinolite is monoclinic, a = 9.84, b = 18.05, c = 5.27, β = 104.7°, Z = 2; space group \(C\dfrac{2}{m}\); point group \(\dfrac{2}{m}\).
Habit
Actinolite typically forms needles or blades – either radiating or in parallel aggregates – or columnar masses.
Structure and Composition
Actinolite is the name given to green amphiboles with compositions intermediate between tremolite, Ca2Mg5Si8O22(OH)2, and Fe-actinolite, Ca2Fe5Si8O22(OH)2. Mn, Al, F, and Cr are sometimes present in minor amounts. Actinolite has the same structure as tremolite and other calcic amphiboles.
Occurrence and Associations
Actinolite is characteristic of medium-grade metamorphosed mafic rocks. It is one of the minerals that give greenschists their characteristic color. Associated minerals typically include albite, epidote, chlorite, and quartz.


Varieties
Byssolite is the name given to fibrous actinolite; Figure 14.130 shows an example. Nephrite, a mineral that may be called jade if gemmy, is an Na-Al variety of actinolite. Figure 14.131 show several different artifacts made from nephrite.
Related Minerals
All amphiboles are structurally similar. Actinolite is closely related to tremolite, Ca2Mg5Si8O22(OH)2, the other calcic amphibole end member.
Hornblende (K,Na)0-1(Ca,Na,Fe,Mg)2(Mg,Fe,Al)5(Si,Al)8O22(OH)2
Origin of Name
From the German word horn (horn) and blenden (blind), referring to its luster (like the horn of an animal) and its lack of value (blenden means deceiving).


Hand Specimen Identification
The two photos show typical specimens of hornblende, the most common amphibole. It is generally vitreous, black, has typical amphibole habit, and near 60o cleavage angle. It is occasionally confused with augite, black pyroxene, but augite has a near 90o cleavage angle. In the absence of compositional information, we use the name hornblende for any black amphibole.
The euhedral crystals seen in Figure 14.132 are attractive but not typical for hornblende. More commonly it occurs in anhedral crystals like those seen in Figure 14.133.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 3.0 to 3.5 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/translucent |
| color | black or less commonly dark green |
| streak | white |
Properties in Thin Section
Hornblende may be various shades of brown, green, blue-green, or yellow-brown in thin section. Moderate to strong pleochroism is typical. Cross sections may be pseudohexagonal or diamond shaped. It may appear superficially like biotite, but has two good cleavages, and generally higher birefringence. 56° and 124° cleavage angles distinguish it from pyroxenes. Biaxial (-), α = 1.65 , β = 1.66, γ = 1.67, δ = 0.02, 2V = 50° to 80°.
Crystallography
Hornblende is monoclinic, a = 8.97, b = 18.01, c = 5.33, β = 105.75°, Z = 2; space group \(C\dfrac{2}{m}\); point group \(\dfrac{2}{m}\).
Habit
Hornblende may be massive or prismatic and is sometimes bladed, columnar, or fibrous. Euhedral crystals are often prismatic with a pseudohexagonal cross section. {100} contact twins are common.
Structure and Composition
Hornblende structure is similar to other amphiboles, except that a large site between tetrahedral chains, vacant in most of them, is partly occupied by Na+ or K+. Thus, hornblende contains seven octahedral cations, and additional Na+ and/or K+ in 10-12 fold coordination.
Hornblende composition varies greatly. Many end members have names; some of the more commonly used ones are:
edenite Ca2NaMg5(AlSi7)O22(OH)2
ferro-edenite Ca2NaFe5(AlSi7)O22(OH)2
pargasite Ca2NaMg4Al(Al2Si6)O22(OH)2
ferro-pargasite Ca2NaFe4Al(Al2Si6)O22(OH)2
tschermakite Ca2Mg3Al2(Al2Si6)O22(OH)2
ferrro-tschermakite Ca2Fe3Al2(Al2Si6)O22(OH)2
tremolite Ca2(Mg,Fe)2Si8O22(OH)2
ferro-actinolite Ca2(Fe,Mg5)Si8O22(OH)2
glaucophane Na2Mg3Al2Si8O22(OH)2
kaersutite Ca2Na(Mg,Fe)4Ti(Al2Si6)O22(OH)2
Besides compositional variations described by the end members listed above, some hornblende varieties include F– or O2- substituting for (OH)–, or Fe3+ substituting for Fe2+
Occurrence and Associations
Hornblende is found in many kinds of igneous rocks covering a wide range of composition. It is usually associated with plagioclase and may coexist with quartz or with mafic minerals such as pyroxene or olivine. It is especially common in igneous rocks of intermediate composition such as syenite or diorite. Hornblende is also found in metamorphosed mafic rocks, especially in amphibolites (that typically contain hornblende and plagioclase as dominant minerals).
Related Minerals
All of the amphiboles are closely related in composition and structure. Hornblende has a more variable composition than most of the others.
Glaucophane Na2Mg3Al2Si8O22(OH)2
Origin of Name
From Greek words meaning “to appear bluish.”


Hand Specimen Identification
Association with other high-pressure minerals in blueschists, often fibrous habit, near 60° cleavage angle, and blue color are distinctive of glaucophane and related Na-amphiboles crossite and riebeckite.
Figure 14.134 shows a blueschist that is mostly made of blue glaucophane. If you enlarge the view, you can see that some small grains of green pyroxene (omphacite) are present, too. This is a typical glaucophane occurrence; this amphibole rarely forms coarse crystals. Figure 14.135 shows the same two minerals, glaucophane and omphacite, but the glaucophane forms slender blue needles. Figure 8.82 is a photo of glaucophane with fuchsite (Cr-rich mica) from France.
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 3.1 to 3.2 |
| cleavage/fracture | near 60° cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | blue or sometimes light gray |
| streak | white to very light blue |
Properties in Thin Section
Glaucophane may be difficult to tell from other blue amphiboles (such as riebeckite or crocidolite). It is colorless to blue or violet in thin section and often strongly pleochroic. Interference colors may range up to low second order, but are sometimes masked by mineral color. It exhibits typical amphibole cleavage and often forms fine prisms or needles with diamond-shaped cross sections. Biaxial (-), α = 1.66 , β = 1.67, γ = 1.65, δ = 0.01 to 0.02, 2V = 0° – 50°.
Crystallography
Glaucophane is monoclinic, a = 9.78, b = 17.80, c = 5.30, β = 103.76°, Z = 2; space group \(C\dfrac{2}{m}\); point group \(\dfrac{2}{m}\).
Habit
Acicular, asbestiform, columnar, or fibrous habit characterizes glaucophane.
Structure and Composition
Glaucophane has a structure similar to the calcic amphiboles (see tremolite structure). Although glaucophane has end-member composition Na2Mg3Al2Si8O22(OH)2, most natural samples contain substantial Fe and Al. Fe2+ replaces Mg2+ and Fe3+ replaces Al3+. If Fe2+ and Fe3+ replace most of the Mg2+ and Al3+, the amphibole becomes riebeckite. In riebeckite, some Na+ enters a normally vacant interlayer site between tetrahedral chains. Compositions intermediate between glaucophane and riebeckite are called crossite.
Occurrence and Associations
Glaucophane is a high-pressure metamorphic mineral characteristic of the blueschist facies. Other blueschist minerals commonly found with glaucophane include jadeite, lawsonite, and aragonite.
Related Minerals
Glaucophane is similar in structure and chemistry to all amphiboles, but in particular the sodic amphiboles: riebeckite, Na2Fe3Fe2Si8O22(OH)2; eckermannite, NaNa2Mg4AlSi8O22(OH)2; and arfvedsonite, NaNa2Fe5Si8O22(OH)2. In the latter two, substantial Na+ occupies a normally unoccupied interlayer site between tetrahedral chains.
Anthophyllite (Mg,Fe)7Si8O22(OH)2
Origin of Name
From the Latin word anthophyllum, meaning “clove leaf,” referring to this mineral’s color.


Hand Specimen Identification
Anthophyllite is characterized by its whitish to clove-brown color, usual prismatic or acicular habit, and prismatic cleavages with a 54° to 55° cleavage angle. It is, however, difficult to distinguish from other light-colored amphiboles such as gedrite, grunerite, or cummingtonite. Some samples of anthophyllite are fibrous to asbestiform.
Figure 14.136 shows a sample from Finland that contains stellate (star-like) anthophyllite needles that radiate from points. Figure 14.137 shows a different specimen from Finland that contains sprays of anthophyllite needles that are more subparallel. In this second photo, green actinolite accompanies the light colored anthophyllite. Figure 3.65, from Chapter 3, is a photo closeup of anthophyllite needles.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 2.9 to 3.2 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {210}, poor (100)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | brown to green |
| streak | white |
Properties in Thin Section
In thin section, anthophyllite is colorless to pale brown or green and may be weakly pleochroic. It shows typical amphibole cleavage angles (56° and 124°) and up to second-order interference colors. It is difficult to tell from gedrite, but parallel extinction distinguishes it from clinoamphiboles. Biaxial (+ or -), α = 1.60, α = 1.62, γ = 1.63, δ = 0.03, 2V = 65° to 90°.
Crystallography
Anthophyllite is orthorhombic, a = 18.56, b = 18.01, c = 5.28, Z = 4; space group \(P\dfrac{2}{n}\dfrac{2}{m}\dfrac{2}{a}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Anthophyllite crystals are prismatic, fibrous, bladed, or columnar with diamond-shaped cross sections.
Structure and Composition
Anthophyllite is part of a solid-solution series extending from Mg7Si8O22(OH)2 toward Fe7Si8O22(OH)2. Although compositionally identical to the monoclinic cummingtonite–grunerite amphiboles, anthophyllite is orthorhombic. Anthophyllite is usually Mg-rich. Al and Na may be present in anthophyllite; if Al content is great enough, the amphibole is called gedrite. The structure of anthophyllite is similar to that of other amphiboles.
Occurrence and Associations
Anthophyllite is found in low-grade Mg-rich metamorphic rocks where it may be associated with cordierite. It is sometimes secondary after high-temperature minerals such as pyroxene and olivine, and is common in some serpentinites.
Varieties
Amosite is the name given to asbestiform anthophyllite.
Related Minerals
Anthophyllite is similar to all the other amphiboles, especially gedrite, (Mg,Fe,Mn)2(Mg,Fe,Al)5(Si,Al)8O22(OH)2, and holmquistite, Li2(Mg,Fe)3Al2Si8O22(OH)2. It is polymorphic with cummingtonite.
14.1.3.3 Pyroxenoids
Pyroxenoid Group Minerals
wollastonite CaSiO3
rhodonite MnSiO3
pectolite NaCa2(SiO3)3H
Pyroxenoids are chain silicates having structures similar, but not identical, to pyroxenes. In pyroxene chains, SiO4 tetrahedra zigzag back and forth. In common pyroxenoids, every other tetrahedron has the same orientation; the chains have a repeat distance of two tetrahedra, approximately 5.2 Å. In pyroxenoids, repeat distances involve three or more tetrahedra, sometimes in complex arrangements. Ca2+, Mn2+, and other cations, bonded to unshared chain oxygens, occupy distorted octahedral sites between chains. The less regular structures of pyroxenoids have less symmetry than pyroxenes; pyroxenoids are triclinic, whereas pyroxenes are monoclinic or orthorhombic.
Wollastonite CaSiO3
Origin of Name
Named after W. H. Wollaston (1766–1828), who discovered palladium and rhodium, invented the reflecting goniometer, and developed the camera lucida.



Hand Specimen Identification
Wollastonite’s restricted occurrence in high-temperature metacarbonates, white color, and common splintery or bladed habit due to two perfect cleavages make it distinctive. Tremolite has many features in common with wollastonite, but wollastonite can be distinguished by its two perfect cleavages about 84° apart (c.f., 56° in tremolite). Pectolite may be confused with wollastonite; both are typically white with similar lusters and habits.
Figure 14.138 shows a large piece of wollastonite containing subparallel blades. In Figure 14.139, wollastonite blades/needles form sprays. Figure 14.140 is a classic sample of marble from the Adirondack Mountains, New York, that contains stubby blades/tabs of white wollastonite, equant grains of green diopside, and minor red garnet.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 3.1 |
| cleavage/fracture | near 90o cleavage angle, perfect (100) and (001), good (102)/uneven |
| luster/transparency | silky, vitreous/translucent |
| color | white |
| streak | white |
Properties in Thin Section
In thin section, wollastonite is clear and has low birefringence. It has two good-perfect cleavages in cross section and one in prismatic section, and it has near-parallel extinction. Wollastonite is distinguished from tremolite, pectolite, and diopside by its lower birefringence. Biaxial (-), α = 1.620 , β = 1.632, γ = 1.634, δ = 0.014, 2V = 39°.
Crystallography
Wollastonite is triclinic, a = 7.94, b = 7.32, c = 7.07, α = 90.03°, β = 95.37°, γ = 103.43°, Z = 4; space group \(P\overline{1}\); point group \(\overline{1}\).
Habit
Wollastonite typically forms cleavable masses or fibrous aggregates. Tabular or prismatic crystals are also common.
Structure and Composition
Wollastonite, generally close to 100% CaSiO3, may contain minor Mn substituting for Ca. Its structure is similar to other pyroxenoids and to pyroxenes (see the introduction to pyroxenoid group minerals and enstatite).
Occurrence and Associations
Wollastonite is common in high-grade marbles and other calcareous metamorphic rocks, especially contact metamorphic rocks. Common associated minerals are calcite, dolomite, tremolite, epidote, garnet, diopside, and vesuvianite.
Related Minerals
Wollastonite and other pyroxenoids are related to pyroxenes in both chemistry and structure. Other pyroxenoids include rhodonite, MnSiO3; pectolite, NaCa2(SiO3)3H; and bustamite, (Mn,Ca,Fe)SiO3. Pseudowollastonite, a high-temperature polymorph, is similar to wollastonite, and several other wollastonite polymorphs are known.
Rhodonite MnSiO3
Origin of Name
From the Greek word rhodon, meaning “rose,” in reference to rhodonite’s color.


Hand Specimen Identification
Rhodonite is one of the few pink minerals and has a nearly perfect 90° cleavage angle. Occurence with other Mn-minerals aids identification. It is occasionally confused with rhodochrosite but is harder and has a different habit.
Figure 14.141 shows typical anhedral rhodonite. Its pink color and hardness are keys to identification. Figure 14.142 shows a spectacular specimen of euhedral rhodonite and quartz. The color of the rhodonite in this specimen is uncommon but there are few other minerals that can have this hue. Figure 10.65 is a photo of another rhodonite crystal with the same vivid color.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 3.5 to 3.7 |
| cleavage/fracture | near 90o cleavage angle, perfect {110}, perfect {110}/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | pink, occasionally red; weathers to dark-colored Mn-oxide |
| streak | white |
Properties in Thin Section
Rhodonite is weakly pleochroic, colorless to light pink in thin section; maximum interference color is first-order yellow. Inclined extinction, high index of refraction, and low birefringence help identify it. Biaxial (+), α = 1.717 , β = 1.720, γ = 1.730, δ = 0.013, 2V = 63° to 76°.
Crystallography
Rhodonite is triclinic, a = 7.68, b = 11.82, c = 6.71, α = 92.35°, β = 93.95°, γ = 105.67°, Z = 2; space group \(P\overline{1}\); point group \(\overline{1}\).
Habit
Cleavable masses or discrete grains are typical of rhodonite; thick tabular crystals, such as those seen in Figure 14.142, above, are uncommon but are diagnostic.
Structure and Composition
The rhodonite structure is similar to that of other pyroxenoids (see the introduction to the pyroxenoid group minerals). Rhodonite always contains some Ca substituting for Mn. If Ca content is great enough, it becomes bustamite. Fe and Zn may be present as well.
Occurrence and Associations
Rhodonite is found in manganese deposits and some iron formations. It is often found with Zn-minerals. Other associated minerals include the Mn-minerals rhodochrosite, bustamite, pyrolusite, tephroite, zincite, willemite, and also calcite and quartz.
Related Minerals
Rhodonite is similar in composition and structure to other pyroxenoids, especially pyroxmangite, (Mn,Fe)SiO3, and bustamite, (Mn,Ca,Fe)SiO3. Pyroxmangite, however, contains less Ca and more Fe, and bustamite contains significantly more Ca.
Pectolite NaCa2(SiO3)3H
Origin of Name
From the Greek word pectos, meaning “well put together.”


Hand Specimen Identification
Light color, occurrence in fibrous radiating masses, and common silky or translucent luster help identify pectolite. It has two cleavages at near 90o, and breaks into sharp acicular fragments when cleaved. Pectolite is occasionally confused with wollastonite or zeolites.
The two photos seen here are samples of pectolite from New Jersey. In Figure 14.143, the crystals are in clearly seen radiating sprays. In Figure 14.144, they are in radiating sprays too, but the radiating crystals have formed globular balls and have a botryoidal appearance.
Physical Properties
| hardness | 5 |
| specific gravity | 2.9 |
| cleavage/fracture | near 90o cleavage angle, two perfect prismatic {100}, perfect {001} |
| luster/transparency | silky/translucent |
| color | white |
| streak | white |
Properties in Thin Section
Pectolite is colorless in thin section, has moderate birefringence and relief, and shows two perfect cleavages with parallel extinction. Biaxial (+), α = 1.59 , β = 1.61, γ = 1.63, δ = 0.04, 2V = 35° to 63°.
Crystallography
Pectolite is triclinic, a = 7.99, b = 7.04, c = 7.02, α = 90.05°, β = 95.28°, γ = 102.47°, Z = 2; space group \(P\overline{1}\); point group \(\overline{1}\).
Habit
Pectolite is typically fibrous or acicular. Acicular radiating crystals forming compact masses, such as those seen in the two photos above, are common.
Structure and Composition
Pectolite is similar in structure to wollastonite. Twisted chains of SiO4 tetrahedra run parallel to the b-axis and are connected by Na and Ca in octahedral coordination. Pectolite may contain small amounts of Fe, K, or Al.
Occurrence and Associations
Pectolite is usually a secondary mineral, resembling zeolites in appearance and occurrence. It is typically found as crusts, in cavities, or along joints in basalt. Associated minerals include zeolites, calcite, and prehnite. It is occasionally found as a primary mineral in alkalic igneous rocks or in calcic metamorphic

Varieties
Larimar (Figure 14.145) is a gemmy blue variety of pectolite found only in the Dominican Republic.
Related Minerals
Pectolite is structurally and chemically related to other pyroxenoids and pyroxenes.


