14.1.1: Silicate Class - Framework Silicates
- Page ID
- 18633
In the sections that follow, we look systematically at the most common minerals that belong to each of the groups listed above. We start with minerals of the Silicate Mineral Class and the Framework Silicate Subclass.
14.1.1.1 Silica Group Minerals
Silica Group Minerals
quartz SiO2
cristobalite SiO2
tridymite SiO2
coesite SiO2
stishovite SiO2
The silica group minerals are framework silicates with composition SiO2 (silica). They belong to the Silicate Mineral Class. Below, we consider the most important of these minerals in more detail; they are listed in the table on the right.
The most common silica mineral is quartz. Besides quartz, other silica polymorphs include cristobalite, tridymite, coesite, and stishovite. The different silica polymorphs vary in the way SiO4 tetrahedra join to form a three-dimensional framework. All except stishovite have structures based on individual SiO4 tetrahedra linked at their vertices by “bridging” oxygen.
Adding complications, quartz, tridymite, and cristobalite have low-temperature (α) and high-temperature (β) polymorphs with slightly different atomic arrangements. Coesite and stishovite do not. Additionally, scientists have synthesized several other SiO2 polymorphs in the laboratory. Although mineralogists have described more than half a dozen silica polymorphs, only common quartz, properly called low quartz, or α-quartz, exists in substantial amounts; it is the second most abundant mineral in Earth’s crust.
Because they have different atomic arrangements, the different SiO2 polymorphs vary in symmetry. For example, low quartz is trigonal, high quartz is hexagonal, low tridymite is orthorhombic, low cristobalite is tetragonal, and coesite is monoclinic.
As discussed in Chapter 6, structural variations among the SiO2 polymorphs reflect the different conditions under which they form. Although low quartz is the only stable SiO2 polymorph under normal Earth surface conditions, some rocks contain metastable stishovite, coesite, cristobalite, or tridymite.
For more general information about silica minerals and their stability fields, consult Chapter 6: Igneous Rocks and Silicate Minerals.
Quartz (α-quartz) SiO2
Origin of Name
From German quartz of unknown origin.
Hand Specimen Identification
Hexagonal symmetry, vitreous luster, hardness (H = 7), lack of cleavage, and conchoidal fracture usually serve to identify quartz. It commonly appears clear and translucent, but not always. Quartz is sometimes confused with calcite, beryl, cordierite, or feldspars.




When euhedral, quartz‘s hexagonal prismatic habit can be distinctive. These four photos show examples. You can click on any photo to see an enlarged version.

Figures 14.1 and 14.2 are photos of clusters containing classic clear hexagonal quartz crystals. Crystal clusters of this sort most often occur in geodes or vugs. Figure 14.3 shows a similar cluster that contains amethyst (purple quartz). Figure 14.4 is a photo of a zoned crystal with amethyst at its top and clear quartz at its bottom. Figure 14.5 shows less perfect and “dirtier,” but perhaps more typical, euhedral quartz crystals. The hexagonal symmetry is still clear to see.



The photos above are of euhedral crystals, but most natural quartz crystals are anhedral or subhedral. These three figures show examples. Hexagonal symmetry is not apparent, but vitreous luster, lack of cleavage, and hardness still identify these specimens as quartz.
Physical Properties
| hardness | 7 |
| specific gravity | 2.65 |
| cleavage/fracture | no cleavage or parting; brittle/conchoidal fracture |
| luster/transparency | vitreous/transparent |
| color | most commonly colorless; also white, milky; less commonly purple, pink, yellow, brown, or black |
| streak | white |
Properties in Thin Section
In thin section, quartz is distinguished by low relief, low birefringence (maximum interference colors are gray), lack of color, lack of cleavage, lack of visible twinning, lack of alteration, usually anhedral character, and undulatory extinction. Uniaxial (+); ω = 1.544, ε = 1.553, δ = 0.009.
Crystallography
Low quartz forms trigonal crystals and has a hexagonal unit cell. a = 4.913, c = 5.405, Z = 3; space group R312 or R322 ; point group 32.

Habit
Quartz may be massive or may form prismatic crystals. Crystals belong to crystal class 32, but appear to have 6-fold symmetry. Common crystals are six-sided prisms terminated by rhombohedrons, sometimes appearing to be hexagonal dipyramids. The quartz crystal in Figure 14.9 is an example (but in the movie Congo, it was misidentified as a diamond). Prism faces often show horizontal growth striations. Rare forms include trapezohedra. Most, if not all, quartz is twinned. Two kinds of twins, Dauphinè and Brazil, are common, but normally cannot be seen with the naked eye.
Structure and Composition
Quartz is always essentially pure SiO2 but may contain trace amounts of other elements. It may be compositionally zoned, as seen above in Figure 14.4. The atomic arrangement consists of a three-dimensional framework of SiO4 tetrahedra, with all oxygens shared by two tetrahedra. At 1 atm, upon heating to 573°C, (1,063°F) minor changes in bond angles cause low quartz (α-quartz) to change into high quartz (β-quartz) with crystal symmetry 622; it inverts to low quartz when cooled.
Occurrence and Associations
Quartz is a common and essential ingredient in many sedimentary, metamorphic, and igneous rocks. It dominates in sandstone and quartzite. It occurs in all silicic metamorphic and igneous rocks. It also dominates in beach sands, many soils, and other sediments.
Varieties
Coarsely crystalline varieties of quartz include citrine (yellow to orange), amethyst (purple), rose quartz (pink), smoky quartz (yellow-brown to black), and milky quartz (milky white). Examples of some of these are in the photos above.
Fibrous microcrystalline varieties of quartz include many types of chalcedony, such as carnelian (red), sard (brown), chrysoprase (apple green), agate (banded or variegated), and onyx (white and gray bands). Jasper (iron red), chert (light gray), and flint (dull dark color) are granular microcrystalline varieties of quartz.


Some specimens of quartz contain inclusions of other minerals. For example, Figure 14.10 is a photo of quartz that contains needles of rutile, and Figure 14.11 shows quartz that contains needles of tourmaline.
Related Minerals
More than a half dozen SiO2 polymorphs exist, but low quartz is the only common one. Opal is an amorphous variety of SiO2 that contains some H2O. Figures 3.28, 7.62, and 9.63 show some examples of opal.
Cristobalite SiO2
Origin of Name
Named after occurrence at Cerro San Cristóbal, Mexico.

Hand Specimen Identification
Cristobalite is generally difficult to identify in hand specimen. X-ray or optical techniques are required. Cristobalite is sometimes confused with zeolites if it appears to be filling vugs or openings in basalt.
Occasionally, cristobalite appears as white spheroids or snowflakes in obsidian. Figure 14.12 is a photo of a large block of obsidian containing many white spheroids of white cristobalite. Figure 4.5, from Chapter 4, shows cristobalite snowflakes in obsidian.
Physical Properties
| hardness | 6.5 |
| specific gravity | 2.33 |
| cleavage/fracture | no cleavage or parting; brittle/conchoidal fracture |
| luster/transparency | vitreous/translucent to transparent |
| color | most commonly colorless |
| streak | white |
Properties in Thin Section
Crystal habit, low birefringence, and moderate negative relief characterize cristobalite in thin section. Low cristobalite: Uniaxial (-), ω = 1.489, ε = 1.482, δ = 0.007.
Crystallography
Low cristobalite is tetragonal, a = 4.97, c = 6.93, Z = 4;space group P43212; point group 422. High cristobalite is cubic, a = 7.13, Z = 8; space group \(F\dfrac{4_{1}}{d}\overline{3}\dfrac{2}{m}\); point group \(\dfrac{4}{m}\overline{3}\dfrac{2}{m}\).
Habit
Cubic, octahedral, or coarse aggregates typify cristobalite. Although low cristobalite crystals belong to crystal class 422, they often appear as small octahedra or globby aggregates.
Structure and Composition
Cristobalite, always nearly pure SiO2, may contain minor amounts of Al3+ and alkalis. As with quartz, the structure is based on a three-dimensional framework of SiO4 tetrahedra. The difference between cristobalite, quartz, and other SiO2 polymorphs is the way in which the tetrahedra are linked. Mineralogists have described two cristobalite polymorphs: low cristobalite (α-cristobalite) is tetragonal, high cristobalite (β-cristobalite) is cubic.
Occurrence and Associations
Cristobalite is only found in high-temperature silicic extrusive igneous rocks. Rapid cooling may keep it from changing into the more stable, low quartz. Typically it occurs as small spherical grains or aggregates in vugs, as misty inclusions in volcanic glass, or as principal components in fine-grained ground mass. It is associated with other high-temperature minerals including sanidine and tridymite.
Related Minerals
SiO2 polymorphs include quartz, cristobalite, tridymite, coesite, and stishovite.
Tridymite (low tridymite) SiO2
Origin of Name
From Greek for threefold, a reference to its habit of forming compound crystals of three individuals or triangular wedge-shaped crystals.
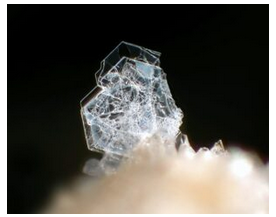
Hand Specimen Identification
Tridymite is usually sufficiently fine grained that X-ray or optical measurements are needed for identification. It is sometimes confused with zeolites. Samples with grains coarse enough to see easily are rare, but they are distinctive. Figures 14.13 is an example, and Figure 4.48 from a previous chapter shows a different example.
Physical Properties
| hardness | 6 to 7 |
| specific gravity | 2.28 |
| cleavage/fracture | no cleavage/conchoidal fracture |
| luster/transparency | vitreous/translucent to transparent |
| color | most commonly colorless |
| streak | white |
Properties in Thin Section
In thin section, tridymite can be distinguished by its low birefringence, low refractive index (RI), moderate relief, and typical habit often showing wedge-shaped twins. Low tridymite: biaxial (+), α = 1.478, β = 1.479, γ = 1.481, δ = 0.003, 2V = 70°.
Crystallography
Low tridymite is orthorhombic, a = 9.9, b = 17.1, c = 16.3, Z = 64; space group P222; point group 222
Habit
Characteristic habit includes wedge-shaped crystals in vesicles or on the walls of cavities of volcanic rocks. Crystals belong to crystal class 222, but often appear as twinned pseudomorphs after high tridymite (6/m2/m2/m).
Structure and Composition
Tridymite may contain minute amounts of Al3+ and alkalis. Its structure consists of sheets of SiO4 tetrahedra joined together by bridging oxygens. Three tridymite polymorphs are known (low, middle, and high tridymite).
Occurrence and Associations
Tridymite is found in high-temperature silicic igneous rocks, where it commonly associates with other high-temperature minerals, including sanidine and cristobalite. It is also found in some stony meteorites and lunar basalts.
Related Minerals
SiO2 polymorphs include quartz, cristobalite, tridymite, coesite, and stishovite.
Coesite SiO2

Origin of Name
Named after chemist Loring Coes, Jr., who first described it in detail.
Hand Specimen Identification
Coesite, only stable at very high pressure, is found in meteor craters and is generally very fine-grained and unidentifiable in hand specimen. Synthetic coesite can be made in the laboratory; the crystals in Figure 14.14 are examples.
Physical Properties
| hardness | 7 to 8 |
| specific gravity | 2.93 |
| cleavage/fracture | none/conchoidal |
| luster/transparency | vitreous/transparent |
| color | colorless |
| streak | white |
Properties in Thin Section
Biaxial (+), α = 1.59, β = 1.60, γ = 1.60 d = 0.01, 2V = 64o
Crystallography
Coesite is monoclinic, a = 7.17, b = 12.33, c = 7.17, β = 120.0o, Z = 16; space group \(C\dfrac{2}{c}\); point group \(\dfrac{2}{m}\).
Habit
Crystals are usually thin tabs, invisible without a microscope.
Structure and Composition
Coesite’s structure consists of a very dense three-dimensional network of SiO4 tetrahedra. In some ways, the structure is similar to that of feldspars.
Occurrence and Associations
Coesite, a rare high-pressure polymorph of SiO2, is known only from meteor impact craters and some rare xenoliths and other rocks of deep crust or mantle origin.
Related Minerals
SiO2 polymorphs include quartz, cristobalite, tridymite, coesite, and stishovite.
Stishovite SiO2
Origin of Name
Named after Sergey M. Stishov, an early investigator of high-pressure SiO2 polymorphs.

Hand Specimen Identification
Stishovite only exists as very fine grains and is not readily identifiable. Occurrence in meteor impact craters gives a hint to its identity. Like coesite, stishovite is sometimes synthesized in a laboratory. Figure 14.15 is a photo of synthetic crystals.
Physical Properties
| hardness | 7 to 8 |
| specific gravity | 4.30 |
| cleavage/fracture | {110}/conchoidal |
| luster/transparency | vitreous/transparent |
| color | colorless |
| streak | white |
Properties in Thin Section
Uniaxial (+), ω = 1.799, ε = 1.826, δ = 0.027.
Crystallography
Stishovite is tetragonal, a = 4.18, c = 2.66, Z = 2; space group \(P\dfrac{4_{2}}{m}\dfrac{2_{1}}{n}\dfrac{2}{m}\); point group \(\dfrac{4}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Stishovite has a variable habit depending on how it forms.
Structure and Composition
Stishovite is always nearly pure SiO2. The structure is extremely dense and resembles the structure of rutile. In stishovite, Si4+ is in octahedral coordination, in contrast with the other SiO2 polymorphs.
Occurrence and Associations
Stishovite, a rare, high-pressure polymorph of SiO2, is known only from meteor impact craters.
Related Minerals
SiO2 polymorphs include quartz, cristobalite, tridymite, coesite, and stishovite.
14.1.1.2 Feldspar Group Minerals
|
Alkali Feldspar Series End members: |
Plagioclase Feldspar Series End members: |
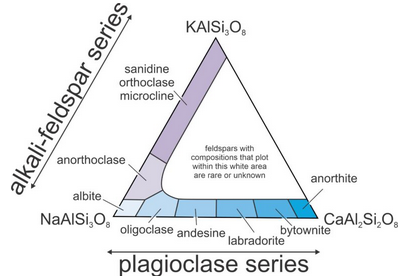
Feldspars are the most abundant minerals in Earth’s crust. Their compositions vary but may be described with the general formula (Ca,Na,K)(Si,Al)4O8.
Feldspar structures are based on SiO4 and AlO4 tetrahedra linked to form a three-dimensional framework. They form two series that share one end-member composition: the alkali feldspar series (mainly NaAlSi3O8 –KAlSi3O8) and the plagioclase (mainly NaAlSi3O8– CaAl2Si2O8) series. Figure 14.16 depicts the series and gives specific names for feldspars of different compositions. Feldspars with compositions that plot in the white center region of the triangle are rare or do not exist.
Distinguishing among the different kinds of feldspars in a hand specimen can be difficult. All are hard, vitreous, show about the same cleavage (if it shows at all). Alkali feldspars, in particular, have variable colors; plagioclase may also. Plagioclase is generally white, but alkali feldspars may be white, too. Occasionally, feldspars may be confused with other light-colored hard minerals, including spodumene or amblygonite.
Feldspars commonly form simple or polysynthetic twins, or combinations. Both contact and penetration twins are possible. Sometimes twinning is visible with the naked eye, but often requires a microscope to be seen. Twin laws include Carlsbad, Baveno, Mannebach, albite, and pericline.
For more general information about the feldspar group, see the Section 6.4.2 of Chapter 6.
Alkali Feldspar
Alkali feldspars range in composition from albite (NaAlSi3O8) to orthoclase (KAlSi3O8). They also contain minor amounts of anorthite (CaAl2Si2O8). Mixing between alkali feldspar and plagioclase is limited, but greatest at high temperature. Figure 14.16, above, shows the typical compositional range for feldspars that equilibrated at normal temperatures.
K-rich feldspar may be either of three polymorphs: sanidine, orthoclase, or microcline. The three differ in the way SiO4 and AlO4 tetrahedra are distributed in their structures. Sanidine, the high-temperature polymorph, is most disordered; microcline, the low-temperature polymorph, is most ordered. Orthoclase has intermediate and somewhat variable ordering. Na-rich feldspar, too, has different polymorphs; they include monalbite at high temperature and low-albite at low temperature.
Original high-temperature alkali feldspars commonly reequilibrate with cooling. Thus sanidine may reorder to become orthoclase or microcline, and monalbite may change into low albite. Often, however, even if crystals reorder, they maintain their original high-temperature shapes.

At high temperatures, alkali feldspars form complete solid solutions between monalbite (the high-temperature NaAlSi3O8 polymorph) and sanidine (high-temperature KAlSi3O8). Intermediate compositions are often termed anorthoclase. At intermediate and low temperatures, however, anorthoclase is unstable because a solvus limits solid solutions. Consequently, high-temperature alkali feldspars commonly exsolve (unmix) to form two compositions. One composition is KAlSi3O8-rich and the other is NaAlSi3O8-rich. This produces perthite, an exsolved feldspar that contains orthoclase-rich and albite-rich stripes called exsolution lamellae. The specimen in Figure 14.17 is a good example of perthite. The K-rich and Na-rich lamellae have slightly different colors. The Na-rich (albite) lamellae always contain some anorthite component, and thus are equivalent in composition to plagioclase.

Figure 14.17 shows perthite from the Black Hills, South Dakota. The lamellae run vertically in the photo and are distinguished by contrasting shades of white and gray. Figure 14.18 is less typical, but also shows perthite. The specimen contains visible millimeter-thick stringers/lamellae of feldspars with two different compositions. The alkali feldspar lamellae are stained red because alkali feldspar often contains small amounts of hematite.
In the sections that follow, we look into the most important alkali feldspar end members in more detail.
Orthoclase KAlSi3O8
Origin of Name
From the Greek word orthos (right angle) and klasis (to break), referring to this mineral’s perpendicular cleavages.

Hand Specimen Identification
The luster, hardness, and color of orthoclase may be similar to other feldspars, but (in contrast with plagioclase) orthoclase is frequently tan, pink, or flesh colored (Figure 14.19); plagioclase is usually white. Orthoclase has cleavage planes that meet at about 90o, like other feldspars. But, orthoclase does not show twin striations like plagioclase does. Association with other felsic minerals also helps identify this mineral.
The photo in Figure 4.41 shows orthoclase that displays classic penetration twins. The combination of tan color with this sort of twinning identifies this mineral. Figures 6.39 (orthoclase from Minas Gerais, Brazil) and 10.61, from previous chapters, show two other examples of orthoclase that have tannish hues.
Distinguishing orthoclase from its polymorphs, microcline and sanidine, can be very difficult without X-ray or optical data. It is sometimes confused with calcite or corundum but can be distinguished by its hardness, which falls between the other two.
Physical Properties
| hardness | 6 |
| specific gravity | 2.56 |
| cleavage/fracture | cleavage angle at about 90o; perfect (001), good (010), poor {110}/uneven |
| luster/transparency | vitreous/transparent/translucent |
| color | colorless, sometimes a bit pink |
| streak | white |
Properties in Thin Section
In thin section, orthoclase has low birefringence, moderate relief, and resemblance to quartz. However, it has negative relief, is biaxial, and is often clouded by fine-grained alteration. It is distinguished from sanidine by 2V and from microcline by its lack of plaid twinning. Biaxial, α = 1.521, β = 1.525, γ = 1.528, δ = 0.007, 2V = 60° to 65°.
Crystallography
Orthoclase is monoclinic, a = 8.56, b = 12.99, c = 7.19, β = 116.01°, Z = 4; space group \(C\dfrac{2}{m}\); point group \(\dfrac{2}{m}\).

Habit
Orthoclase crystals are prismatic, stubby to elongate, and may be flattened or doubly terminated. Penetration twins and contact twins are common. Figure 14.20 shows typical twinned orthoclase.
Structure and Composition
The structure of orthoclase consists of a three-dimensional framework of SiO4 and AlO4 tetrahedra. K+ ions occupy available holes between the tetrahedra. Most orthoclase contains some Na replacing K; complete solid solution between orthoclase and albite (NaAlSi3O8) is possible only at high temperature. Some orthoclase contains small amounts of CaAl replacing NaSi.
Occurrence and Associations
Orthoclase is common in many kinds of silicic igneous rocks, sediments such as arkoses, and a variety of metamorphic rocks. Quartz and micas are typically associated minerals.
Varieties
If orthoclase shows opalescence, we call it moonstone, Figure 14.21 shows an example.
Related Minerals
The principal K-feldspar polymorphs are sanidine (high-temperature form), orthoclase (moderate temperature form), and microcline (low-temperature form). They differ in the way SiO4 and AlO4 tetrahedra are arranged in their structure. Several other related minerals are known: adularia is a colorless, transparent form of K-feldspar that forms prismatic crystals.
Sanidine (K,Na)AlSi3O8
Origin of Name
From the Greek word sanis (tablet) and idios (appearance), referring to this mineral’s typical habit.

Hand Specimen Identification
Sanidine may be difficult to tell from other feldspars, but its restricted occurrence in felsic volcanic rocks and association with other high-temperature minerals are helpful diagnostic tools. In contrast with other alkali feldspars, orthoclase tends to be colorless, and often transparent to translucent.
Certain identification requires X-ray analysis or thin sections. Plagioclase and microcline have different kinds of twins than sanidine, and sanidine never shows twin lamellae. Most sanidine probably changes into orthoclase or microcline with cooling and time, but it usually retains its original monoclinic crystal shape.
Physical Properties
| hardness | 6 |
| specific gravity | 2.56 |
| cleavage/fracture | cleavage angle at about 90o; perfect (001), good (010) |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless or white most of the time |
| streak | white |
Properties in Thin Section
In thin section, sanidine appears similar to orthoclase but has greater 2V. Carlsbad twins may divide crystals into halves. Manebach and Baveno twins may also be present. Biaxial (-), α = 1.521, β = 1.525, γ = 1.528, δ = 0.007, 2V varies depending on structure.
Crystallography
Sanidine is monoclinic, a = 8.56, b = 13.03, c = 7.17, β = 116.58°, Z = 4; space group \(C\dfrac{2}{m}; point group \dfrac{2}{m}.
Habit
Sanidine crystals are prismatic, may be tabular or elongate, and often have a square cross section. Carlsbad twins are common.
Structure and Composition
Similar to orthoclase, the structure of sanidine consists of a three-dimensional framework of SiO4 and AlO4 tetrahedra. In sanidine the two kinds of tetrahedra are randomly distributed in the structure, while in orthoclase they are partially ordered. K+ ions occupy available holes between the tetrahedra. Na may replace K; complete solid solution between sanidine (KAlSi3O8) and albite (NaAlSi3O8) is possible at high temperature.
Occurrence and Associations
Sanidine occurs in silicic igneous rocks but is restricted to rocks that have cooled quickly. If cooling is slow, orthoclase will be present instead. Typical occurrences are as phenocrysts in rocks such as trachyte or rhyolite.
Related Minerals
Related minerals include the other KAlSi3O8 polymorphs, orthoclase and microcline, and the plagioclase series.
Microcline KAlSi3O8
Origin of Name
From the Greek word micros (small) and klinein (to lean), referring to this mineral’s cleavage angles being close to 90°.



Hand Specimen Identification
Hardness (H = 6), vitreous/pearly luster, feldspar cleavage, habit, and association help identify microcline, but it may be hard to distinguish from other feldspars, especially orthoclase, its polymorph. Because they all share similar properties, X-ray or optical measurements may be needed for certain identification of feldspars, especially alkali feldspars.
Microcline comes in several different colors. The large tan crystals seen in Figure 14.23 are typical, as are the green-colored crystals seen in Figure 14.24. This bluish-green color is a key to identification but is less common than more mundane colors. Microcline with this color is called amazonite. Figure 14.25 shows another example of microcline. The specimen is from Brazil; the photo contains white microcline accompanied by gray translucent quartz.
Physical Properties
| hardness | 6 |
| specific gravity | 2.56 |
| cleavage/fracture | cleavage angle at about 90o; perfect (001), good (010) |
| luster/transparency | pearly, vitreous/translucent to subtranslucent |
| color | colorless or white, green, salmon-pink |
| streak | white |

Properties in Thin Section
Microcline’s tartan plaid, also called cross-hatched, twinning (a combination of albite and pericline twinning) is its most diagnostic characteristic in thin section (Figure 14.26). Plagioclase may show two sets of twins at about 90°, but in plagioclase the twins have sharp parallel boundaries, while in microcline they pinch and swell. Microcline is biaxial (-), α = 1.518, β = 1.524, γ = 1.528, δ = 0.010, 2V = 77°-84°.
Crystallography
Microcline is triclinic, a = 8.58, b = 12.96, c = 7.21, α = 89.7°, β = 115.97°, γ = 90.87°, Z = 4; space group P1; point group 1.
Habit
Prismatic, stubby to elongate, crystals are typical for microcline. It is also commonly found in cleavable masses or irregular grains. Twins, both contact and penetration, may be present but are normally only visible with a microscope.
Structure and Composition
The structure of microcline is similar to the structures of orthoclase and sanidine, but the SiO4 and AlO4 tetrahedra are more regularly ordered, leading to less symmetry. Ordering decreases with increasing temperature of formation: low microcline has complete tetrahedral ordering. Intermediate and high microcline are less well ordered. A solvus limits solid solutions between microcline and albite, NaAlSi3O8, at low temperatures.
Occurrence and Associations
Microcline is common in many kinds of silicic igneous rocks, sediments such as arkoses, and a variety of metamorphic rocks. It easily forms from sanidine or orthoclase as rocks cool from high
temperatures. Quartz and micas are typically associated minerals.

Varieties
When colored green, microcline is given the name amazonite (see Figure 14.27 here and Figure 14.24, above).
Related Minerals
Related minerals include the other KAlSi3O8 polymorphs, orthoclase and microcline, and the plagioclase series.
Plagioclase (solid solution feldspar)

Plagioclase feldspars are mostly solid solutions of albite (NaAlSi3O8) and anorthite (CaAl2Si2O8). They commonly contain lesser amounts of orthoclase (KAlSi3O8), especially at high temperatures. See Figure 14.16 At medium and high temperatures, plagioclase may have any composition between albite and anorthite.
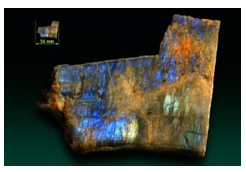
At low temperatures, variable ordering and several small solvi lead to complications not reflected in Figure 14.16. One of the complications is that intermediate-composition plagioclase (called labradorite) may exsolve. This exsolution commonly produces a variety of feldspar that display a play of colors called schiller, or labradorescence. Schiller often looks like colors that may appear on an oil slick. Figure 14.28 shows an example. Figure 6.51 shows additional examples of labradorite.
In the sections that follow, we look at the properties of the two end-member plagioclases, albite and anorthite, but most properties of intermediate compositions are quite similar.
Albite NaAlSi3O8
Origin of Name
From the Latin word albus, meaning “white.”


Hand Specimen Identification
Vitreous luster, hardness (H = 6), cleavage angle near 90o, association, and fine polysynthetic twinning help identify albite and other Na-rich plagioclase. If not twinned, it may be difficult to tell from alkali-feldspar. Distinguishing albite from other plagioclase cannot be done precisely without detailed X-ray or optical data.
The albitic feldspar in Figure 14.29 contains fine twin lamellae; they appear as parallel lines that go from the lower-right to the upper left in the photo. The albite-rich plagioclase in Figure 14.30 show two cleavages and has a typical blocky appearance.
Figures 3.63, 6.37, and 10.63 from previous chapters show other photos of albite.
Physical Properties
| hardness | 6 |
| specific gravity | 2.62 |
| cleavage/fracture | cleavage angle at about 90o; perfect (001), good (010), poor {110}/uneven |
| luster/transparency | pearly, vitreous/translucent |
| color | colorless, white, gray, or green |
| streak | white |
Properties in Thin Section
In thin section, albite and other composition plagioclase show no color, have low relief, and exhibit up to 1st-order gray interference colors. Plagioclase may appear similar to K-feldspars and superficially similar to quartz. However, cleavage, biaxial character, and “zebra stripes” caused by polysynthetic twinning usually serve to identify albite and other plagioclase. Biaxial (+), α = 1.527, β = 1.531, γ = 1.538, δ = 0.011, 2V = 77°.
Crystallography
Albite is triclinic, a = 8.14, b = 12.79, c = 7.16, α = 93.17°, β = 115.85°, γ = 87.65°, Z = 4; space group \(P\overline{1}\); point group \(P\overline{1}\).
Habit
Masses or subhedral grains are common for albite and other plagioclase. Rare euhedral crystals are prismatic, tabular, or bladed. Most crystals are twinned according to the pericline law, and some are twinned by the albite law. Albite twins give albite the characteristic polysynthetic twinning that is often visible as fine striations in hand specimen and as stripes in thin section.
Structure and Composition
Albite is an end member of both the plagioclase and the alkali feldspar series. As with K-feldspar, ordering of AlO4 and SiO4 tetrahedra decreases with increasing temperature, leading to minor changes in structure. Low albite’s structure is similar to that of low microcline; high albite’s structure is more disordered. At very high temperature a completely disordered albite, called monalbite because it is monoclinic, is stable. At all but the lowest temperatures, complete solid solution exists between albite and the other plagioclase end member, anorthite, CaAl2Si2O8. Albite, and other plagioclase, also form limited solid solutions with orthoclase, KAlSi3O8.
Occurrence and Associations
The most abundant minerals of Earth’s crust, plagioclase is found in a wide variety of igneous, metamorphic, and, less commonly, sedimentary rocks. Most compositions are intermediate between albite and anorthite, but compositions approaching end members are known. Albite, defined as plagioclase consisting of greater than 90% NaAlSi3O8, is found in silicic igneous rocks such as granite, syenite, trachyte, or rhyolite, where it associates with quartz and orthoclase.

Varieties
Cleavelandite (Figure 14.31) is a form of albite typified by curved plates; it is found in pegmatites. Opalescent varieties of albite or other plagioclase are called moonstone.
Related Minerals
Albite is closely related to the other, more calcic plagioclase, and to alkali feldspars (orthoclase, sanidine, and microcline).
Anorthite CaAl2Si2O8
Origin of Name
From the Greek word meaning oblique, in reference to anorthite’s crystal shape.


Hand Specimen Identification
Vitreous luster, hardness (H = 6), cleavage angle near 90o, and association, help identify anorthite, but it can be extremely difficult to tell from other feldspars. Several kinds of twinning are common (see albite habit). Albite twins, if present, may be difficult to see in hand specimen.
Most anorthite-rich feldspars occur in mafic igneous rocks. Figure 14.32 is a photo of basalt with anorthite crystals. It comes from near the summit of Mt. Vesuvius, Italy. Euhedral anorthite crystals like the ones seen in this photo are rare. Figure 14.33 shows a more common occurrence: light-colored bytownite (anorthite containing significant albite component) crystals in a basalt from Cumbria, England. Bytownite is plagioclase that is 70-90% anorthite (see Figure 14.16).
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 2.76 |
| cleavage/fracture | cleavage angle at about 90o; perfect (001), good (010), poor {110}/uneven |
| luster/transparency | pearly, vitreous/translucent |
| color | white or sometimes gray |
| streak | white |
Properties in Thin Section
In thin section, anorthite is similar to other plagioclase. Biaxial (-), α = 1.577, β = 1.585, γ = 1.590, δ = 0.013, 2V = 78°.
Crystallography
Anorthite is triclinic, a = 8.17, b = 12.88, c = 14.16, α = 93.33°, β = 115.60°, γ = 91.22°, Z = 8; space group \(P\overline(1)); point group \(P\overline(1)).
Habit
Anorthite is common as cleavable masses or irregular grains. Euhedral crystals are rare. They may be prismatic, tabular, or bladed and are frequently twinned according to the same laws as albite. When present, albite twins give calcic plagioclase characteristic polysynthetic twinning, but the width of the twins is usually greater than is common for albitic plagioclase.
Structure and Composition
Anorthite is the calcic end member of the plagioclase series, but the name is also used for any plagioclase containing > 90% CaAl2Si2O8. Its structure is similar to those of albite and orthoclase. As with the other feldspars, the ordering of AlO4 and SiO4 tetrahedra decreases with increasing temperature, leading to minor changes in structure. Complete solid solution of anorthite with albite, NaAlSi3O8, is possible at all but very low temperatures. Minor solid solution with orthoclase, KAlSi3O8, is common.
Occurrence and Associations
Anorthite, found primarily in mafic igneous rocks, is rarer than other plagioclase. In igneous rocks, it associates with amphibole, pyroxene, or olivine. It is occasionally found in metamorphosed carbonates.
Related Minerals
Anorthite is structurally and compositionally related to the other, more sodic plagioclase, and to the potassic feldspars (orthoclase, sanidine, and microcline).
14.1.1.3 Feldspathoid Group Minerals
Feldspathoid Group Minerals
analcime NaAlSi2O6•H2O
leucite KAlSi2O6
nepheline (Na,K)AlSiO4
Feldspathoid minerals are similar to feldspars in many ways, but they contain more Al and less Si. They occur in igneous rocks that contain less SiO2 than normal for the most common igneous rocks.
Like feldspars, feldspathoids have structures based on three-dimensional frameworks of AlO4 and SiO4 tetrahedra; alkalis occupy holes between the tetrahedra. Al:Si ratios vary from 1:1 in nepheline, NaAlSiO4, to 1:4 in the rare feldspathoid petalite, LiAlSi4O10. Feldspathoids are closely related to zeolites; the distinction between the two groups is hazy. The major difference is that zeolite structures contain large cavities or open channels and usually contain molecules of H2O.
The most important feldspathoids are leucite, nepheline, and analcime, although analcime is often considered a zeolite because it contains molecular H2O. Sodalite, Na3Al3Si3O12•NaCl, and haüyne, Na3CaAl3Si3O12(SO4), are sometimes grouped with the feldspathoids but have a cage structure similar to the structures zeolites.
For more general information about the feldspathoid group, see the relevant section in Chapter 6.
Analcime NaAlSi2O6•H2O
Origin of Name
From the Greek word analkimos (weak), referring to its weak pyroelectric character.


Hand Specimen Identification
Light color, equant crystals (if euhedral), occurrences in vugs or cavities, and association help identify analcime, but it may be difficult to tell from leucite. Because of its typical crystal shape, it is occasionally confused with garnet, although garnets are generally much more strongly colored. Figure 14.34 shows very large spherical analcime crystals from Martinique, and Figure 14.35 shows similar crystals from Ireland.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 2.26 |
| cleavage/fracture | poor cubic {100}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | color white, gray, pink |
| streak | white |
Properties in Thin Section
In thin section, analcime is colorless and exhibits low negative relief and low birefringence. It may be confused with leucite, which has higher indices of refraction, or with sodalite, which is usually slightly bluish. Isotropic, n = 1.482.
Crystallography
Analcime is cubic, a = 13.71, Z = 16; space group I41/a32/d; point group 4/m32/m.
Habit
Analcime typically forms distinct euhedral crystals. Trapezohedrons and cubes are common forms, often in combination. Massive and granular aggregates are also known. Sometimes crystals appear to be spherical.
Structure and Composition
The framework structure of analcime consists of AlO4 and SiO4 groups joined to make rings of four, six, or eight tetrahedra. Rings align, producing channels that hold H2O molecules. The channels can hold additional absorbed ions or groups. Na+ ions occupy nonchannel sites between rings. Minor substitution of K or Cs for Ca and of Al for Si are common. Analcime forms a limited solid solution with pollucite, (Cs,Na)2(AlSi2O6)2•H2O.
Occurrence and Associations
Analcime is found in cavities in basalt and as a primary mineral in alkalic igneous rocks such as Na-rich basalt or syenite. In cavities, it is associated with zeolites, calcite, or prehnite.
Related Minerals
Analcime is similar in structure to zeolites such as wairakite, Ca(Al2Si4O12)2H2O, and to leucite, KAlSi2O6.
Leucite KAlSi2O6
Origin of Name
From the Greek word leukos, meaning “white,” in reference to leucite’s color.


Hand Specimen Identification
Occurrence in Si-poor, K-rich volcanic rocks, light color, crystal habit if pseudocubic, and color help identify leucite. It may be confused with analcime, but leucite typically forms as a matrix mineral whereas analcime forms in cavities.
The most common occurrences of leucite are in alkalic volcanic rocks such as the one seen above in Figure 14.36, above. The white crystals are leucite. Figure 14.37 show rare euhedral crystals of leucite from a classic locality in Brazil.
| hardness | 5.5 to 6 |
| specific gravity | 2.48 |
| cleavage/fracture | indiscernible, poor {100}, poor (001)/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white or sometimes gray |
| streak | white |
Properties in Thin Section
Low relief, gray interference colors, and lamellar or concentric twins help identify leucite. Uniaxial (+), ω = 1.508, ε = 1.509, δ = 0.001.
Crystallography
Leucite is tetragonal, a = 13.04, c = 13.85, Z = 16; space group \(I\dfrac{4_{1}}{a}\); point group \(dfrac{4}{m}\).
Habit
Although tetragonal at low temperatures, leucite normally has the form of its high-temperature cubic polymorph. Trapezohedral crystals are typical. Polysynthetic twinning may give faces fine striations.
Structure and Composition
Leucite’s structure consists of a framework of 4-, 6-, and 8-membered rings of AlO4 and SiO4 tetrahedra. K+ ions occupy half the available sites between the rings. Leucite is generally close to end-member composition, although small amounts of Fe, Na, and other alkalis may be present.
Occurrence and Associations
Leucite is a rare mineral found in Si-poor, K-rich volcanic rocks. It is never found with quartz.
Related Minerals
Leucite is isostructural with pollucite, (Cs,Na)(AlSi2O6)2•H2O. Chemically, it is closely related to orthoclase, KAlSi3O8; kaliophilite, KAlSiO4; and to analcime, NaAlSi2O6•H2O. A cubic polymorph of leucite exists above 605°C (1,120 °F).
Nepheline (Na,K)AlSiO4
Origin of Name
From the Greek word nephele, meaning “cloud,” because crystals turn cloudy when immersed in acid.


Hand Specimen Identification
Occurrence in alkaline igneous rocks, blocky habit, clear or whitish color, lack of cleavage, and greasy luster help identify nepheline. It may be confused with feldspar or quartz but is softer. Occasionally it is confused with apatite. These two photos show typical nepheline of two slightly different colors.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 2.60 |
| cleavage/fracture | indiscernible, poor {100}, poor (001)/subconchoidal |
| luster/transparency | subvitreous to vitreous, greasy/sometimes translucent |
| color | white, colorless, turbid |
| streak | white |
Properties in Thin Section
Low birefringence and relief, and common alteration (to zeolites, sodalite, muscovite, or kaolinite), identify nepheline. It is distinguished from the feldspars by its uniaxial nature and from quartz by its optic sign. Uniaxial (-), ω = 1.540, ε = 1.536, δ = 0.004.
Crystallography
Nepheline is hexagonal, a = 10.01, c = 8.41, Z = 8; space group P63 ; point group 6.
Habit
Massive, compact, and embedded grains of nepheline are common. Crystals are short and prismatic with six or twelve sides.
Structure and Composition
The structure of nepheline is similar to the structure of tridymite, but every other Si is replaced by Al, and Na occupies large sites between Al and Si tetrahedra. All natural nepheline contains some K substituting for Na, but a solvus exists between nepheline and kalsilite, KAlSiO4, at temperatures below 1,000°C (1,830 °F). Exsolution, similar to perthite (see the general discussion of alkali feldspars, above), is common.
Occurrence and Associations
Nepheline is characteristic of some Si-poor igneous rocks, such as syenite. It is found with feldspars, apatite, cancrinite, sodalite, zircon, and biotite.
Related Minerals
Nepheline is isostructural with tridymite. It has a high-temperature polymorph above 900°C (1,650 °F). It forms a solid solution with kalsilite, KAlSiO4, and is chemically related to kaliophilite, KAlSiO4. Cancrinite, (Na3Ca2)2CO3(Si3Al3O12)•2H2O, is similar to nepheline in many ways and occurs in the same type of rocks.
14.1.1.4 Scapolite Series Minerals
Scapolite End Members
marialite Na4(AlSi3O8)3Cl
meionite Ca4(Al2Si2O8)3(CO3,SO4)
Scapolite is a solid-solution metamorphic mineral with compositions related to the feldspars, but with structures more closely related to nepheline. The two principal end members are marialite, Na4(AlSi3O8)3Cl, equivalent in composition to albite plus halite; and meionite, Ca4(Al2Si2O8)3(CO3,SO4), equivalent in composition to anorthite plus calcite/anhydrite. Complete solid solution between the two end members is possible; F and OH may replace Cl and CO3.
Scapolite (Na,Ca)4(Si,Al)14O24(Cl,CO3,SO4)
Origin of Name
From the Greek word skapos, meaning stalk, in reference to its common woody appearance.


Hand Specimen Identification
Scapolite is characterized by prismatic crystals with square cross sections and two cleavages at 45° to each other. Commonly, euhedral scapolite crystals are translucent like the ones shown in Figure 14.40. Massive samples often have a distinct woody or fibrous appearance, like the specimens seen in the figure. Scapolite may be confused with feldspar when not transparent and gemmy and if its prismatic habit is not evident.
Scapolite is sometimes transparent and gemmy, like the purple scapolite in Figure 14.41.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 2.55 |
| cleavage/fracture | good {100}, poor {110}/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | variable, typically yellow or light brown but also purple, rarely clear |
| streak | white |
Properties in Thin Section
Scapolite may appear similar to feldspars but is uniaxial and has different cleavage. Uniaxial (-), ω = 1.540, ε = 1.536, δ = 0.004.
Crystallography
Scapolite is tetragonal, a = 12.11, c = 7.56, Z = 2; space group \(I\dfrac{4}{m}\); point group \(\dfrac{4}{m}\).
Habit
Crystals of scapolite are typically tetragonal prisms, often woody looking and translucent. Massive varieties are common.
Structure and Composition
Scapolite‘s structure is closely related to that of nepheline, consisting of AlO4 and SiO4 joined in a three-dimensional framework. The structure contains two types of holes for anions and anionic groups: one holds Na and Ca; the other Cl or CO3. CaAl substitutes for NaSi freely; SO4 and F may substitute for Cl and CO3. The two dominant scapolite end members are marialite, Na4(AlSi3O8)3Cl, and meionite, Ca4(Al2Si2O8)3(CO3,SO4). Natural scapolite has intermediate compositions. Chemically, scapolite is equivalent to plagioclase plus CaCO3, NaCl, or CaSO4. Minor K, OH, and F may be present.
Occurrence and Associations
Most scapolite occurrences are in calcic metamorphic rocks: marbles, marls or mafic gneisses, and amphibolites. Rare occurrences are reported from igneous rocks. Associated minerals include plagioclase, clinopyroxene, hornblende, apatite, garnet, and titanite. Scapolite easily alters into low-temperature minerals.

Varieties
Scapolites with compositions between meionite and marialite are sometimes called wernerite.
Scapolite gemstones are not abundant, but come from many places around the world. Figure 14.42 shows a rough scapolite crystal, and the faceted and polished gem produced from a similar crystal. There is quite a difference! Typical scapolite gems are either yellow, like the one on the right in Figure 14.42, or purple (see Figure 14.41, above).
1.1.5 Zeolite Group Minerals
Zeolite Minerals
natrolite Na2Al2Si3O10•2H2O
chabazite CaAl2Si4O12•6H2O
heulandite CaAl2Si7O18•6H2O
stilbite CaAl2Si7O18•7H2O
sodalite Na3Al3Si3O12•NaCl
Zeolites are a large and important group of secondary minerals, typically formed by alteration of minerals in igneous rocks. Only five are considered in detail here, but more than 40 natural species are known, and many equivalent phases have been synthesized. Almost all of them have compositions more-or-less equivalent to hydrated feldspars.
All zeolites are framework silicates, containing a three-dimensional network of SiO4 and AlO4 tetrahedra linked to form channels, cages, rings, or loops. Alkalis or alkaline earths occupy large sites in the structures. Some zeolites have 4-, 6-, 8-, or 10-member tetrahedral rings. Others contain complex polyhedra resulting in cage-like openings. Unlike other framework silicates, zeolites contain large open cavities and channels in their structures that permit cations and H2O molecules to pass in and out without disruption. Consequently, zeolites are used as molecular sieves in water softeners and other industrial applications.
Crystal symmetry and morphology vary between species. Different zeolites are cubic, tetragonal, orthorhombic, hexagonal, or monoclinic, and habits may be fibrous, tabular or platy, prismatic, or equant. Although sodalite is sometimes grouped with the feldspathoids, it is here grouped with the zeolites because it has a cage-like structure with 4- and 6-member silica rings.
For more general information about zeolites, consult the zeolites section in Chapter 7.
Natrolite Na2Al2Si3O10•2H2O
Origin of Name
From the Greek word natron, meaning “soda.”
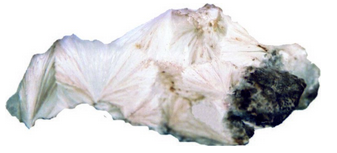

Hand Specimen Identification
The many different zeolites have similar occurrences and may be hard to distinguish. Natrolite typically occurs as white radiating needles or as radial aggregates. Its perfect prismatic cleavage and uneven fracture and association also aid identification. Natrolite may occasionally be confused with aragonite or pectolite.
Figure 14.43 is a photo of a mass of natrolite crystals that appear to be radiating from common points. In Figure 14.44, natrolite crystals are separated acicular needles instead of massive, producing a delicate spray.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 2.23 |
| cleavage/fracture | perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless or sometimes gray |
| streak | white |
Properties in Thin Section
Natrolite may be difficult to distinguish from other zeolites. It has two perfect cleavages, has parallel extinction, is length slow, and has a moderate 2V. Biaxial (+), α = 1.48, β = 1.48, γ = 1.49, δ = 0.012, 2V = 38° to 62°.
Crystallography
Natrolite is orthorhombic, a = 18.30, b = 18.63, c = 6.60; space group Fd2d; point group mm2.
Habit
Acicular crystals, often radiating, are typical for natrolite. Crystals may show vertical striations. Radiating rounded masses are also common. Less commonly, it is fibrous, massive, or granular.
Structure and Composition
Natrolite and all zeolites are framework structures built of AlO4 and SiO4 tetrahedra. The tetrahedra are linked to form chains, cages, rings, or loops. In contrast with some other framework silicates, the zeolite structure contains many large holes and channels, which hold weakly bonded H2O. Slightly smaller holes contain Na, Ca, or K. In natrolite, scolecite, and some other zeolites, the tetrahedral framework has a strong linear fabric parallel to the c-axis. Crystal habit is therefore fibrous or acicular. Natrolite always contains minor amounts of Ca and K and has variable H2O content.
Occurrence and Associations
Natrolite (and most other zeolites) are secondary minerals that most commonly form in cracks or on cavity walls in mafic igneous rocks. It also occurs in altered ash or igneous rocks of other kinds, and rarely as a vein mineral. Natrolite is found with calcite and other zeolites.
Related Minerals
Scolecite, CaAl2Si3O10•3H2O, and thomsonite, NaCa2 Al5Si5O20•6H2O, are closely related to natrolite.
Chabazite CaAl2Si4O12•6H2O
Origin of Name
From the Greek word chabazios, meaning “hail.”


Hand Specimen Identification
Form, color, and association help identify chabazite but it may be difficult to distinguish from other zeolites. Crystals are usually translucent pseudocubic rhombs, similar to many calcite crystals. Chabazite is, however, distinguished from calcite by its poorer cleavage and lack of reaction to HCl.
Chabazite may have any of a number of different colors. Salmon or orange colors, as seen in Figures 14.45 and 14.46, are most common and help with identification. The pseudocubic shapes of the chabazite crystals are clear in both photos. In Figure 14.45, the chabazite is on top of clear heulandite.
Physical Properties
| hardness | 4 to 5 |
| specific gravity | 2.1 |
| cleavage/fracture | poor rhombohedral {101}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless, salmon, pink, orange, or red |
| streak | white |
Properties in Thin Section
Uniaxial (-), ω = 1.484, ε = 1.481, δ = 0.003.
Crystallography
Chabazite crystals are trigonal. a = 13.17, c = 15.06, Z = 6; space group \(R\overline{3}\dfrac{2}{m}\); point group \(\overline{3}\dfrac{2}{m}\).

Habit
Chabazite usually forms simple rhombohedral crystals that may, at first glance, appear cubic. Some crystals are more complicated, showing more than one rhombohedral form or exhibiting twinning. Figure 14.47 is a photo of clear chabazite displaying cyclic twinning.
Structure and Composition
Chabazite is similar in structure to natrolite and other zeolites. Tetrahedra form large cage-like openings, which can hold a variety of loosely bonded ions and molecules. The large openings allow diffusion of some small molecules through the structure. Considerable substitution of Na and K for Ca is common.
Occurrence and Associations
Chabazite is a secondary mineral that forms in cracks or on cavity walls in mafic igneous rocks and as an alteration product in silicic igneous rocks. Less commonly, it occurs in sedimentary and metamorphic rocks. It is typically found with calcite and with other zeolites.
Related Minerals
Several other rare zeolites have structures similar to chabazite’s.
Heulandite (Ca,Na)2-3(Si,Al)18O36•12H2O
Origin of Name
Named for John Henry Heuland, a British mineralogist.



Hand Specimen Identification
Heulandite is generally platy and tabular. It varies greatly in color and may be difficult to distinguish from other vitreous zeolites, especially clinoptilolite. Crystal habit, perfect one direction of cleavage, and common transparency aid identification.
Figure 14.48 shows clear heulandite crystals from Austria. Figure 14.45, above, shows other clear crystals of heulandite, but with chabazite. Figure 14.49 is a photo of green heulandite from India, and Figure 14.50 shows salmon-colored heulandite in a vug. White/creamy stilbite surrounds the heulandite.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 2.15 |
| cleavage/fracture | one perfect (010)/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white, variable, sometimes clear |
| streak | white |
Properties in Thin Section
Heulandite is biaxial (+), α = 1.49, β = 1.50, γ = 1.50, δ = 0.005, 2V = 35°.
Crystallography
Heulandite is monoclinic, a = 17.73, b = 17.82, c = 7.43, β = 116.3°, Z = 4; space group Cm; point group m.
Habit
Heulandite crystals are typically platy with a diamond or modified diamond shape, sometimes described as “coffin-shaped.”
Structure and Composition
The structure of heulandite is similar to that of other zeolites, except that tetrahedra are linked in 6-member rings that align to give a more planar structure than most others. End-member calcic heulandite, called mordenite, is rare. Considerable solid solution towards clinoptiloite, a K-containing zeolite, is typical.
Occurrence and Associations
Heulandite is one of the more common zeolites. It is a secondary mineral, found with calcite and with other zeolites, that forms in cracks or on cavity walls in mafic igneous rocks, and in some metamorphic rocks.
Related Minerals
Other zeolites, including clinoptiloite, (Na,K)Si7Al2O18•6H2O, and stilbite, CaAl2Si7O18•7H2O, have structures similar to heulandite’s.
Stilbite CaAl2Si7O18•7H2O
Origin of Name
From the Greek word stilbein, meaning “to shine,” referring to this mineral’s luster.



Hand Specimen Identification
Sheaf-like aggregates of twinned crystals, one excellent cleavage, pearly and translucent luster, color, and association identify stilbite.
Figure 14.51 is a photo of typical clear to translucent white stilbite crystals. Figure 14.52 is a photo of salmon-colored crystals, also typical, from India. Figure 14.53 shows creamy white stilbite with blue cavansite (a rare calcium vanadate mineral). The photo in Figure 14.50 shows stilbite that has a similar color.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 2.15 |
| cleavage/fracture | perfect (010)/subconchoidal |
| luster/transparency | pearly, vitreous/translucent |
| color | clear, white, gray, tan, or pinkish |
| streak | gray |
Properties in Thin Section
Cruciform twins and parallel or near-parallel extinction help identify stilbite in thin section. Biaxial (-), α = 1.49, β = 1.50, γ = 1.50, δ = 0.010, 2V = 30° to 50°.
Crystallography
Stilbite is monoclinic, a = 13.64, b = 18.24, c = 11.27, β = 129.16°, Z = 8; space group \(C\dfrac{2}{m}\); point group \(\dfrac{2}{m}\).
Habit
Simple crystals of stilbite are extremely rare. Aggregates of twinned crystals, having the appearance of sheaves of grain, are common. Sometimes aggregates appear fibrous. More rarely, stilbite forms crystals displaying cruciform twinning.
Structure and Composition
Stilbite’s structure is similar to heulandite’s. Considerable substitution of Na and K for Ca is common.
Occurrence and Associations
Stilbite is one of the more common zeolites. It is a secondary mineral, found with calcite and with other zeolites, that forms in cracks or on cavity walls in igneous rocks and in some schists associated with hydrothermal ore bodies.
Related Minerals
Stilbite is grouped with heulandite, CaAl2Si7O18•6H2O, and several others because of its chemical and structural similarity.
Sodalite Na3Al3Si3O12•NaCl
Origin of Name
This mineral’s name refers to its sodium content.



Hand Specimen Identification
Color (if blue) and association with other alkalic minerals in alkalic rocks are usually diagnostic for sodalite. If not blue, identification may require chemical tests to tell it from zeolites or analcime. It is sometimes confused with lazulite, another blue mineral, but has different associations.
The photos in Figure 14.54 and 14.55 show typical sodalite samples. Massive anhedral specimens are typical and give little hint of sodalite‘s crystal morphology. The photo in Figure 14.56 shows subhedral crystals of sodalite; subhedral and euhedral crystals of sodalite are uncommon. Sodalite belongs to the cubic crystal system but crystals are generally not cubes.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 2.3 |
| cleavage/fracture | six poor {110} at 60° angles/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | typically blue, rarely whitish |
| streak | white |
Properties in Thin Section
In thin section, sodalite is distinguished by being isotropic and having a low index of refraction and, sometimes, a hexagonal outline. Isotropic, n = 1.485.
Crystallography
Sodalite is cubic, a = 8.87, Z = 2; space group \(P\overline{4}3m\); point group \(\overline{4}3m\).
Habit
Often massive or in embedded grains, sodalite forms rare dodecahedral crystals.
Structure and Composition
The structure of sodalite is similar to many zeolites, containing 4- and 6-member tetrahedral rings but, unlike true zeolites, it contains no molecular water that can be driven off easily. The Al and Si tetrahedral rings are linked to form a framework with cage-like openings that hold Cl and sometimes S or SO4. Sodalite is usually close to end-member composition. Small amounts of K or Ca may also be present.
Occurrence and Associations
Sodalite is associated with nepheline, (Na,K)AlSiO4, cancrinite, (Na3Ca2)(Al3Si3O12)CO3•2H2O, leucite, KAlSi2O6, and with feldspars in Si-poor, alkali-rich igneous rocks.
Varieties
Hackmanite is a sulfurous form of sodalite.
Related Minerals
Sodalite is structurally and chemically related to other zeolites and to cancrinite, (Na3Ca2)2(Al3Si3O12)CO3•2H2O.
14.1.1.6 Other Framework Silicates
Other Framework Silicates
beryl Be3Al2Si6O18
cordierite (Mg,Fe)2Al4Si5O18
Beryl and cordierite, which contain 6-membered tetrahedral rings, are sometimes considered to be ring silicates. They are, however, more properly classified as framework silicates because they have an overall three-dimensional framework of connecting tetrahedra. These two minerals share a common property: open channels in their structures almost always contain some H2O.
Beryl Be3Al2Si6O18
Origin of Name
From the Greek word beryllos, meaning “a blue-green gem.”

Hand Specimen Identification
Beryl is often easily identified because of its vitreous luster, typical blue or green color and hexagonal crystals. Occurrence in pegmatites and, less commonly, granites, also aids identification. Even if not clearly hexagonal, color is often a giveaway (Figure 14.57). It is very hard and has poor or no cleavage. If not euhedral or blue, however, it may be difficult to distinguish from other hard vitreous minerals.
Figure 14.58 shows typical blue beryl in a pegmatite. Associated minerals are quartz and orthoclase. Figure 14.59 is also a photo of blue beryl in a pegmatite, but the beryl stands out more clearly. Black tourmaline, orthoclase, and quartz are also present in this specimen. Figure 14.60 is a photo of emerald (green beryl) with quartz. Figure 14.61 is a photo of beryl crystals that have been removed from a pegmatite; they are subhedral to euhedral hexagonal prisms.




Physical Properties
| hardness | 7.5 to 8 |
| specific gravity | 2.7-2.9 |
| cleavage/fracture | poor (001)/even |
| luster/transparency | vitreous/transparent to translucent |
| color | blue, colorless, variable |
| streak | white |
Properties in Thin Section
Beryl is uniaxial (-), ω = 1.568, ε = 1.562, δ = 0.006. In thin section, beryl may appear similar to quartz, apatite, or topaz, but quartz is (+), apatite has higher relief, and topaz is biaxial.
Crystallography
Beryl is hexagonal. a = 9.23, c = 9.19, Z = 2; space group \(P\dfrac{6}{m}\dfrac{2}{c}\dfrac{2}{c}\); point group \( \dfrac{6}{m} \dfrac{2}{m} \dfrac{2}{m} \).
Habit
Beryl typically forms hexagonal prisms. When present, terminating faces are pinacoids or, more rarely, pyramids. Single crystals are common; columnar aggregates are less so.
Structure and Composition
6-membered tetrahedral rings form sheets that are linked by tetrahedral Be and octahedral Al. Some classification schemes group beryl with true ring silicates such as tourmaline, but in beryl the framework structure is not completely planar and there is much cross linking. Small amounts of Na, Rb, and Li may substitute for Be; minor H2O and CO2 may occupy spaces within the rings.
Occurrence and Associations
Beryl is mostly found in granitic rocks, notably in pegmatites. It may also be found in schists and in rare ore deposits.
Varieties
Beryl can be many different colors due to small amounts of trace elements. Emerald is vivid green; aquamarine is pale blue or greenish blue; morganite is rose colored; heliodor is gold; bixbite is red. The photos below show some of these varieties. The most spectacular examples of beryl are euhedral, like the bixbite, heliodor, and aquamarine examples below. But the emerald specimen is pretty spectacular, too, because of its vivid color.




Related Minerals
Cordierite, (Mg,Fe)2Al4Si5O18, is similar in structure to beryl. Euclase, BeAlSiO4(OH), is another of the rare beryllium silicates.
Cordierite (Mg,Fe)2Al4Si5O18
Origin of Name
Named after P. L. A. Cordier (1777–1861), the French mineralogist who first described the mineral.



Hand Specimen Identification
Prismatic cleavage, color, and association aid identification, but if it does not appear similar to dark blue bottle glass, this mineral is often difficult to distinguish without a microscope. It may be easily confused with quartz in hand specimen and with plagioclase in thin section.
Figure 14.66 shows the most common occurrence of blue cordierite – in a high-grade metamorphic rock with red garnet. Figure 14.67 is an enlarged view of a typical blue anhedral cordierite crystal. Cordierite often has a dark gray or black color like the crystals in Figure 14.68. These crystals may show a hint of blue color and have orthorhombic shapes – which helps identify them as cordierite.
Physical Properties
| hardness | 7 to 7.5 |
| specific gravity | 2.5 to 2.8 |
| cleavage/fracture | fair prismatic (010), poor (100)/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | indigo to gray-blue to gray are most common |
| streak | white |
Properties in Thin Section
In thin section, cordierite may be clear or very pale blue-violet. Lamellar twinning is common, similar to plagioclase twinning, except the twins pinch out. Pleochroic halos around zircon inclusions, when present, are easily seen and diagnostic. Cordierite often alters to a fine-grained mass called pinnite. It may be confused with quartz, feldspar, or nepheline. Uniaxial (+ or -), a = 1.54, β = 1.55, γ = 1.56, δ = 0.02, 2V = 65° to 105°.
Crystallography
Cordierite is orthorhombic. a = 17.13, b = 9.80, lc = 9.35, Z = 4; space group \(C\dfrac{2}{c}\dfrac{2}{c}\dfrac
{2}{m}\); point group \(\dfrac{2}{m}\dfrac{2}{m}\dfrac{2}{m}\).
Habit
Rare, euhedral crystals are short and prismatic, and may appear pseudohexagonal. Twins are common. Cordierite is most often granular, sometimes massive.
Structure and Composition
Cordierite, like beryl, consists of 6-membered tetrahedral rings joined in a three-dimensional framework. Mg2+, Fe2+, and Al3+ link the rings to each other. Hollow channels, sometimes occupied by H2O or alkalis, run parallel to the c-axis. Fe:Mg ratio is variable.
Occurrence and Associations
Cordierite is found as a product of contact or regional metamorphism in high-grade metamorphosed aluminous rocks. Rare occurrences in igneous rocks have been reported. Associated minerals include garnet, sillimanite, spinel, plagioclase, anthophyllite, and orthopyroxene.
Varieties
A high-temperature polymorph of cordierite, indialite, is isostructural with beryl.
Related Minerals
Cordierite is structurally similar to beryl, and is chemically and structurally similar to osumilite, (K,Na)(Fe,Mg)2(Al,Fe)3(Si,Al)12O30•H2O.


