2.3: Mineral Identification
- Page ID
- 2492
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)INTRODUCTION
Minerals can be identified by their physical characteristics. The physical properties of minerals are related to their chemical composition and bonding. Some characteristics, such as a mineral’s hardness, are more useful for mineral identification. Color is readily observable and certainly obvious, but it is usually less reliable than other physical properties.
HOW ARE MINERALS IDENTIFIED?
Mineralogists are scientists who study minerals. One of the things mineralogists must do is identify and categorize minerals. While a mineralogist might use a high-powered microscope to identify some minerals, most are recognizable using physical properties.

Check out the mineral in figure 1. What is the mineral’s color? What is its shape? Are the individual crystals shiny or dull? Are there lines (striations) running across the minerals? In this lesson, the properties used to identify minerals are described in more detail.
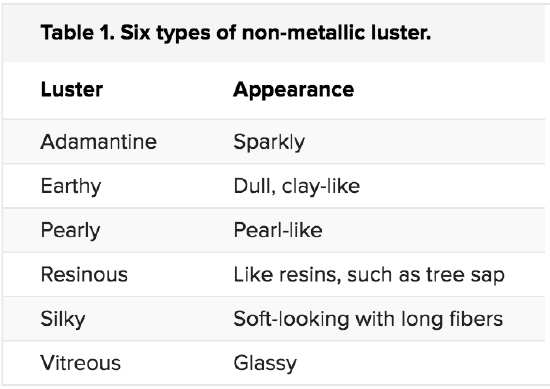
COLOR, STREAK, AND LUSTER
Diamonds are popular gemstones because the way they reflect light makes them very sparkly. Turquoise is prized for its striking greenish-blue color. Notice that specific terms are being used to describe the appearance of minerals.
Color
Color is rarely very useful for identifying a mineral. Different minerals may be the same color. Real gold, as seen in figure 2, is very similar in color to the pyrite in figure 1.

The same mineral may also be found in different colors. Figure 3 shows one sample of quartz that is colorless and another quartz that is purple. A tiny amount of iron makes the quartz purple. Many minerals are colored by chemical impurities.

Streak
Streak is the color of a mineral’s powder. Streak is a more reliable property than color because streak does not vary. Minerals that are the same color may have a different colored streak. Many minerals, such as the quartz in the figure 3, do not have streak.
To check streak, scrape the mineral across an unglazed porcelain plate (Figure 4). Yellow-gold pyrite has a blackish streak, another indicator that pyrite is not gold, which has a golden yellow streak.

Luster
Luster describes the reflection of light off a mineral’s surface. Mineralogists have special terms to describe luster. One simple way to classify luster is based on whether the mineral is metallic or non-metallic. Minerals that are opaque and shiny, such as pyrite, have a metallic luster. Minerals such as quartz have a non-metallic luster. Different types of non-metallic luster are described in table 1.
Can you match the minerals in figure 5 with the correct luster from table 1?

SPECIFIC GRAVITY
Density describes how much matter is in a certain amount of space: density = mass/volume.
Mass is a measure of the amount of matter in an object. The amount of space an object takes up is described by its volume. The density of an object depends on its mass and its volume. For example, the water in a drinking glass has the same density as the water in the same volume of a swimming pool.
Gold has a density of about 19 g/cm3; pyrite has a density of about 5 g/cm3—that’s another way to tell pyrite from gold. Quartz is even less dense than pyrite and has a density of 2.7 g/cm3.
The specific gravity of a substance compares its density to that of water. Substances that are more dense have higher specific gravity.
HARDNESS
Hardness is a measure of whether a mineral will scratch or be scratched. Mohs Hardness Scale, shown in table 2, is a reference for mineral hardness.
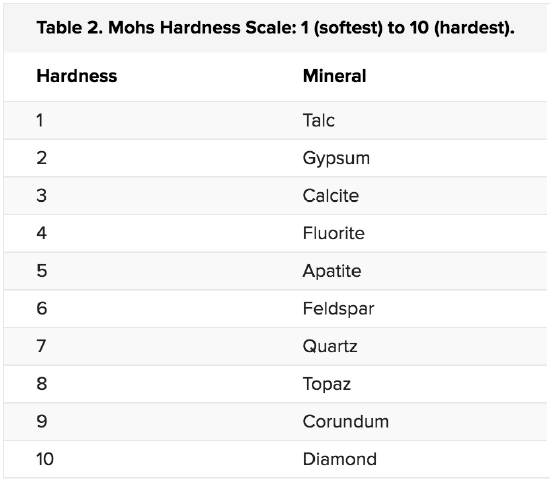
With a Mohs scale, anyone can test an unknown mineral for its hardness. Imagine you have an unknown mineral. You find that it can scratch fluorite or even apatite, but feldspar scratches it. You know then that the mineral’s hardness is between 5 and 6. Note that no other mineral can scratch diamond.
CLEAVAGE AND FRACTURE
Breaking a mineral breaks its chemical bonds. Since some bonds are weaker than other bonds, each type of mineral is likely to break where the bonds between the atoms are weaker. For that reason, minerals break apart in characteristic ways.
Cleavage is the tendency of a mineral to break along certain planes to make smooth surfaces. Halite breaks between layers of sodium and chlorine to form cubes with smooth surfaces (figure 6).
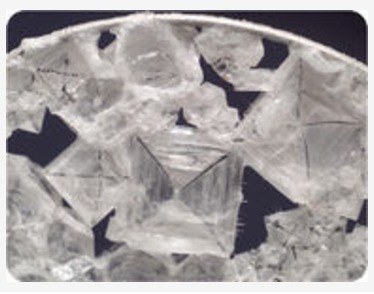
Mica has cleavage in one direction and forms sheets (figure 7).

Minerals can cleave into polygons. Fluorite forms octahedrons (figure 8).

One reason gemstones are beautiful is that the cleavage planes make an attractive crystal shape with smooth faces.
Fracture is a break in a mineral that is not along a cleavage plane. Fracture is not always the same in the same mineral because fracture is not determined by the structure of the mineral.
Minerals may have characteristic fractures (figure 9). Metals usually fracture into jagged edges. If a mineral splinters like wood, it may be fibrous. Some minerals, such as quartz, form smooth curved surfaces when they fracture.

OTHER IDENTIFYING CHARACTERISTICS
Some minerals have other unique properties, some of which are listed in table 3. Can you name a unique property that would allow you to instantly identify a mineral that’s been described quite a bit in this chapter? (Hint: It is most likely found on your dinner table.)

A simple lesson on how to identify minerals is seen in this video.
LESSON SUMMARY
- Minerals have distinctive properties that can be used to help identify them.
- Color and luster describe the mineral’s outer appearance. Streak is the color of the powder.
- A mineral has a characteristic density.
- Mohs Hardness Scale is used to compare the hardness of minerals.
- Cleavage or the characteristic way a mineral breaks depends on the crystal structure of the mineral.
- Some minerals have special properties that can be used to help identify them.
REFLECTION QUESTIONS
- What skill does this content help you develop?
- What are the key topics covered in this content?
- How can the content in this section help you demonstrate mastery of a specific skill?
- What questions do you have about this content?
Contributors and Attributions
Original content from Kimberly Schulte (Columbia Basin College) and supplemented by Lumen Learning. The content on this page is copyrighted under a Creative Commons Attribution 4.0 International license.


