12.3: 12.03- Karst Hydrogeology
- Page ID
- 25565
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
The processes of water infiltration and flow in karst landscapes is distinctly different from that in other landscapes. In the simplest sense, any precipitation falling onto a karst landscape will infiltrate downwards through the soil towards the soil/bedrock contact, and then the water is likely to continue vertically down through the many small fractures or conduits in the karst bedrock (the epikarst) towards a zone of saturation forming a groundwater aquifer (Figure 12.3.2). Surface flow on karst landscapes is limited and occurs only where the karst is covered by impermeable soils (e.g., till) or in periods of heavy rainfall. On the surface of a karst landscape there are some obvious hydrological differences: a general lack of surface drainage or streams, discrete sink points or swallets where streams disappear, and springs where water emerges. Surface streams will only flow over karst when precipitation exceeds what will infiltrates downward through the karst surface. In many cases karst streams are inactive during low flow periods and flow only during flood events. Some of the most spectacular features associated with karst streams are karst canyons, where aggressive water flow has actively cut into soluble bedrock creating steep and sometimes overhanging sidewalls.

Components of a Karst Aquifer
Conceptually, a karst aquifer is a relatively simple system that has a site (or sites) of recharge (where water enters the aquifer), a medium that can store and move water, and a site (or sites) of discharge where water leaves the system (Figure 12.3.3). Recharge of a karst aquifer is carried out at discrete point inputs (such as a swallet) or by diffuse infiltration through soil into the epikarst, where it can be stored and gradually released into the subsurface groundwater system.
In most karst aquifers (i.e., those comprised of well lithified and crystalline limestone) groundwater is primarily stored within fractures and conduits. Groundwater storage in matrix pores is more common in geological young or partially lithified carbonate units (e.g., calcareous dune sands) and other specific types of carbonates such as chalk.
Most of the subsurface flow in karst aquifers occurs along conduits that dominate the groundwater system, and transport water to springs at some predetermined base level. The base level is generally considered as the lowest point to which water can go.

Several important terms are used to define a karst aquifer a vertical sense. The upper surface of the karst landscape is known the epikarst, where solutionally enlarged openings lead gradually down to more confined openings and fractures. In some places discrete vertical/sub-vertical openings (e.g., swallets, shafts, cave entrances) are present on the karst surface and are linked directly to conduits, caves, and other cavities (Figure 12.3.4). Water percolating down through the epikarst and discrete openings eventually reaches the water table – a surface that defines the boundary between the zone of aeration and saturation. The zone of aeration is more commonly known as the vadose zone and is where pore spaces contain both air and water, while the zone of saturation is known as the phreatic zone and is where all pore spaces are water-filled. (Note, that in many cases a straightforward planar water table surface does not define the top of the phreatic zone for any karst landscape. In fact, there are likely to be any number of perched water tables at various levels depending on how conduits and caves are distributed and connected).
In the vadose zone most of the water flow is vertical, while in the phreatic zone most of the flow is sub-horizontal and along conduits. (The upper most part of the phreatic zone is known as the epiphreatic zone (where vadose waters meet phreatic) and is generally regarded as the site of greatest dissolution and conduit/cave development.
The phreatic zone is divided into three sub-zones:- The shallow phreatic (dominated by moderate/fast sub-horizontal groundwater flow),
- The deep phreatic or bathyphreatic (characterised by slower groundwater flow), and
- If the karst unit is deep enough, a lower stagnant phreatic or nothephreatic sub-zone where there is little to no flow.
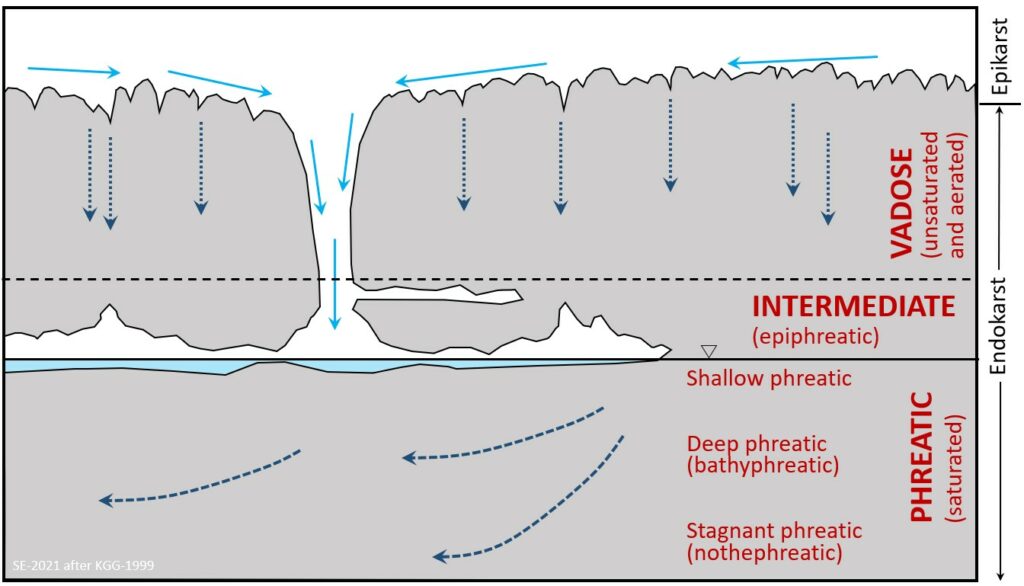
Exercise 12.1 Finding the Zones in Karst
On this cross-section diagram of a karst aquifer label areas where you would expect to see diffuse recharge and where there would be point-source recharge.
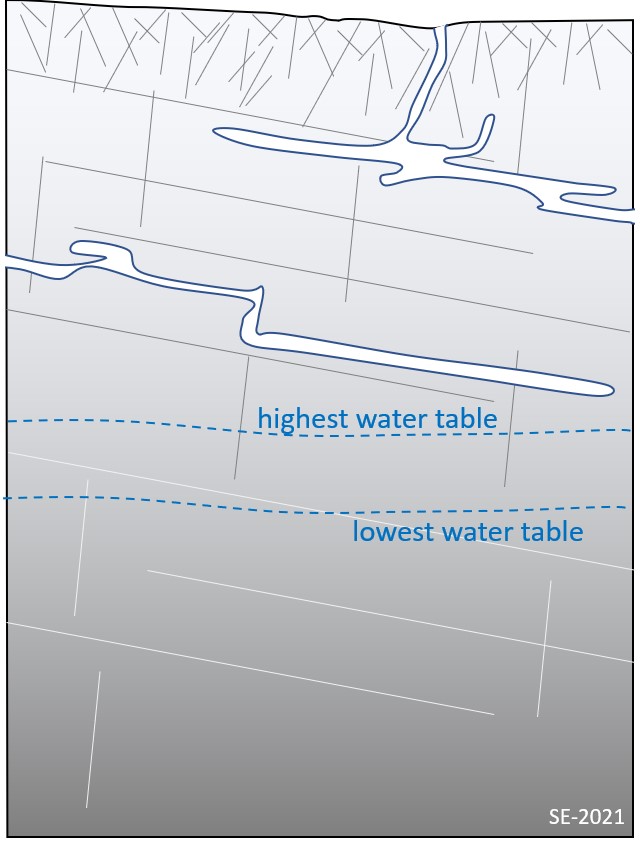
Label the vadose zone, the epiphreatic zone and the phreatic zone.
Exercise answers are provided Appendix 2.
Karst Groundwater Chemistry
Karst groundwater is chemically, quite distinct from other types of groundwater, primarily because of the solutional processes and chemical reactions that take place between the water and the surrounding bedrock. The chemistry of karst waters is controlled by dissolution factors (e.g., rainfall, temperature, soil carbon dioxide), the types of bedrock (limestone, dolomite, gypsum) and the residence time of water within the bedrock. The chemical nature of the water taken from a karst spring can be used along with other data (e.g., water conductivity, dye tracing) to provide information on the groundwater system, including the source of recharge and to whether it comes from point or diffuse inputs. Ions that are typically found in karst waters include Ca2+, Mg2+, K+, Na+, HC03–, SO42-, Cl–, and NO3–. These ions are for the most part byproducts of chemical reactions with the bedrock, and can be measured directly by chemical analysis, or indirectly by measurements of pH (or alkalinity, CO32- and HCO3–) and conductivity (which is a measure of the total dissolved solids content or TDS).
Hardness is the total of the Ca2+ and Mg2+ ions and is a measure of the amount of dissolved limestone. Other measurements that are sometimes taken for karst waters include:
- Dissolved O2 (depleted if removed by biological decay process)
- Dissolved CO2 (greater for water percolating through soil)
- Temperature (usually cooler than surface water)
- Bacteria count (particularly for pollution studies)
- UV fluorescence for organic material (e.g., soil humic or fulvic acids)
Autogenic and Allogenic Recharge of Karst Aquifers
At the broad landscape level, karst is recharged in two ways – by water falling directly onto the karst landscape (autogenic recharge), and by water that that falls on adjacent non-karst landscapes and then enters surface streams, which then in turn flow onto the karst landscape (allogenic recharge) (Figure 12.3.5). Typically, autogenic recharge is where the water falls onto soil or epikarst, becomes enriched in CO2 and provides a primary source for diffuse water input into the underlying aquifer. In some cases, this autogenic recharge flow can be concentrated into point input features such as sinkholes. The characteristics of water from allogenic recharge depend on the upstream conditions, but it generally has a low ion content and carries sediment. In some cases, allogenic waters can be very aggressive (acidic), where derived from wetlands, and on flowing onto the carbonate bedrock causing extensive karstification. On Vancouver Island, it is not uncommon for allogenic streams that drain onto a karst unit to form a line of swallets along the upper boundary of a karst unit.Exercise 12.2 Comparing Groundwater Chemistry
The table below shows the results of the analysis of three groundwater samples from a limestone karst aquifer in England and three from a sandstone aquifer in British Columbia. All of the results are in mg/L.
| Sample | HCO3– | Cl- | SO4-2 | Na+ | K+ | Ca2+ | Mg2+ |
| a | 233 | 20 | 23 | 8 | 1.4 | 74 | 7.6 |
| b | 158 | 24 | 17 | 80 | 0.6 | 5.4 | 1.1 |
| c | 97 | 8 | 14 | 48 | 0.1 | 6.7 | 0.2 |
| d | 271 | 18 | 25 | 6 | 1.4 | 112 | 6.0 |
| e | 290 | 14 | 22 | 6 | 1.1 | 110 | 11 |
| f | 68 | 8.6 | 20 | 126 | 0.4 | 20 | 2.6 |
Based on what you know about limestone and sandstone, indicate which of these 6 samples are likely to be from the karst aquifer and which from the sandstone aquifer.
What are the major differences between the chemistry of the water in these two aquifers?
Exercise answers are provided Appendix 2.
One important concept to understand is the ‘karst catchment’ or drainage area that contributes water to a particular karst unit. The catchment (drainage basin or watershed) for a non-karst landscape is typically defined as all the areas that drain towards the trunk stream and can be easily defined from the surface topography using the heights of land to map out the topographic divides. The limits of a karst catchment are quite different and are not constrained by topographic divides, and in fact they can cross below topographic divides with subsurface water flow along conduits. Delineation of a karst catchment is therefore difficult and may require several techniques to fully determine its extent. One of the most useful techniques for this purpose is dye tracing. Care should be taken to ensure that dye tracing is done under a variety of flow conditions (i.e., flow stages), as catchment areas can vary between low and peak flows depending on the distribution and connections between conduits.Water Storage, Movement, and Discharge of a Karst Aquifer

Several terms are important for understanding groundwater flow (and is relevant to both karst and non-karst aquifers: hydraulic head, hydraulic gradient, and hydraulic conductivity. Hydraulic head is the elevation of a water body above a certain datum (such as sea level) (Figure 12.3.6). This provides the gravitational energy for water to flow downhill. The higher the elevation of the water body above a datum the greater the hydraulic head. The relative change in hydraulic head over a unit of distance is the hydraulic gradient. Hydraulic conductivity can be thought of as the resistance of water flow through a certain rock or material type in a certain amount of time and is measured in m/s. In summary, groundwater flows from a high to low hydraulic head at a rate determined by the hydraulic head and the rock’s resistance to flow (or hydraulic conductivity).

The prime way that water leaves a karst aquifer is by a karst spring. Karst springs represent water that has flowed through a karst aquifer, and typically appear at the surface as a bedrock opening or conduit with flowing water. Karst springs can range from small trickles of water to a raging river ten of metres in width. For the most part karst springs are located at sites of lower elevations along valley floors, sides of lakes or coastal shorelines. In some cases, springs can occur beneath water bodies.
Karst springs differ from those that might occur in other rock types in that they are, for the most part, conduit-fed. Springs can therefore be used to determine many of the physical and chemical characteristics of a karst aquifer. The type of flows at springs can be steady, intermittent, seasonal, or reverse. Springs with steady flow indicate that the aquifer has a significant storage capacity, relative to the amount of water flowing through the system (see Figure 12.3.3). Steady flow springs are sometimes termed as outflow springs, which generally occur near the base level of the aquifer. Seasonal or intermittent flows occur at overflow springs, which are located above outflow springs, and are more active during peak flows or flood events.
When the karst aquifer is confined by an overlying impermeable rock unit, excess hydraulic head can develop and lead to the formation of artesian springs. Springs can also emerge below stream beds, lakes, and the sea, where they are, of course, more difficult to detect. Many karst springs carry excess ions (i.e., are supersaturated) and will form calcareous tufa deposits both around the spring opening and extending as mounds or steps downstream.
Karst Aquifer Investigations
Karst aquifers can be investigated several ways, and this is important prior to any land management decisions. Mapping the extent of the karst catchment can be problematic but it is always required prior to any detailed hydrogeologic analysis. Sites for water input and output need to be determined, as well as any information of subsurface flow paths (e.g., from cave maps). Dye tracing is commonly used to assist in determining the subsurface connections of water flow through conduits and caves.
Karst springs are critical sites for data gathering, as they closely reflect the characteristics of the conduit network and recharge area. For example, springs whose flows fluctuate rapidly with flood events are likely related to allogenic recharge, while springs less susceptible to variations in flow are more likely associated with autogenic recharge. Likewise, the quality of water from springs (e.g., temperature, turbidity, pH, dissolved oxygen, and total dissolved solids) can provide information of possible recharge characteristics and storage or residence times (Figure 12.3.7). Turbid and ion-poor water might suggest allogenic recharge and a short residence time, while alternatively clean and ion-rich water might indicate longer residence and/or autogenic recharge. Continuous data recorders can be set up to measure these many of these characteristics at both predetermined times and during specific events (e.g., floods).
Electrical conductivity measurements using a simple hand-held meter can be used as a rapid mapping tool to determine whether the water in a specific stream has emerged from a karst spring or has been in contact with carbonate bedrock. Generally, the higher the readings the greater the ion content, and more likely the association of the water with carbonate bedrock.
The distribution and orientation of conduits in an aquifer are difficult to determine. Caving and subsurface mapping can be done but is obviously limited to those conduits that are enterable. The use of dye tracing is probably the most effective technique for determining conduit linkages and flow paths. Drilling of boreholes along with pump testing can be also used to evaluate matrix and fracture porosity of a karst unit. Geophysical techniques, such as ground penetrating radar and gravity, can also be used to some extent identify subsurface conduits or openings.
Dye tracing is one of the most important techniques used in the evaluation of karst aquifers – and is one of the most fun to carry out (Figure 12.3.8). The primary goals of dye tracing are to determine flow path connections within a karst aquifer. However, dye tracing can also tell you something about the conduit network within the aquifer, the likely catchment for a spring, and the rates of water flow within the system. Most dye tracing studies use liquid/powdered forms of non-toxic fluorescent dyes (e.g., fluorescein, Rhodamine WT, eosine, and uranine), which are placed at selected injection sites or inputs (e.g., swallets) where water enters an aquifer. (Note, there are also other water tracing methods such as using inert spores, dilute isotopes, or salt, however, non-toxic dyes are usually the most common). Prior to placing the dyes into the injection sites, test collection sites are set up at all the potential outputs, such as springs, reappearing streams, etc.

Impacts to Karst Aquifers and their Remediation
Karst aquifers, like all other groundwater aquifers, can be polluted and impacted in many ways. The prime concerns being both water quantity and quality. Impacts to water quantity can occur for several reasons, particularly where over usage or over pumping from wells occur and pollution materials enter the subsurface. The variable nature of karst aquifers can lead to much confusion as to both input sites and water storage sites within the aquifer. Karst aquifers are particularly sensitive because of their inherent ability to rapidly move pollutants in and through the hydrological system by conduit flow, and the fact that there are many potential linkages or openings between the surface and the subsurface.
Pollution of karst aquifers can come from a variety of sources such as industry, agriculture, urban development, septic systems, and roads. Pollutants can include a variety of metals, organic and non-organic materials, such as nitrates, bacteria, petroleum, salt, sediment. There are two prime groundwater pollution sources that are usually considered – dispersed and point. Remediation of pollutants in a karst aquifer can be done to either eliminate or reduce the contaminants to an acceptable level. In all karst aquifers remediation must consider the three types of porosity: matrix, fracture, and conduit. If most of the pollution material is introduced via conduit flow it can conceivably be flushed through the conduit portion of the karst system with little impact to the rest of the aquifer. Likewise, pollutants introduced via the soil and epikarst may be trapped/stored in the aquifer for a much longer time, and only be flushed out during occasional flood events.
Different parts of the aquifer may require different types of remediation. Remediation techniques can vary from no action (leaving it for natural recovery processes), to extensive treatment of the soil, to pumping and cleaning of the waters. Strategies will depend on pollutant source, type, persistence, host material, flow paths, risks, and resources available. For dispersed sources, the main remediation approach could be to change the practices that caused the pollution, while for point source the probable approach is to remove or contain the pollutant material. Remediation of karst aquifers is a complex, slow and difficult process, that requires careful assessment and evaluation before implementation of the work.
Media Attributions
- Figure 12.3.1 Photo by P. Griffiths, CC BY 4.0
- Figure 12.3.2 Photo by P. Griffiths, CC BY 4.0
- Figure 12.3.3 Steven Earle, CC BY 4.0, after Grimes (2002)
- Figure 12.3.4 Steven Earle, CC BY 4.0, after Grimes (1999)
- Figure 12.3.5 Allogenic and autogenic recharge of a karst landscape by T. Stokes, CC BY 4.0, from Stokes & Griffiths (2011)
- Figure 12.3.6 Photo by P. Griffiths, CC BY 4.0
- Figure 12.3.7 Photo by P. Griffiths, CC BY 4.0
- Figure 12.3.8 Photo by P. Griffiths, CC BY 4.0
- Palmer, A. (2007). Cave geology. Cave Books. ↵


