15.2: Earth’s Temperature
- Page ID
- 6937
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Because the Moon doesn’t have much of an atmosphere, daytime temperatures on the moon are around 224℉ and nighttime temperatures are around -298℉. That is an astonishing 522 degrees of change between the light-side and dark-side of the Moon [2]. This section describes how Earth’s atmosphere is involved in regulating the Earth’s temperature.
Earth’s Energy Budget
Solar radiation arriving at Earth from the Sun is relatively uniform. Energy (or heat) radiates from the Earth’s surface and lower atmosphere back to space. This flow of incoming and outgoing energy is the Earth’s energy budget. For Earth’s temperature to be stable over long stretches of time, incoming energy and outgoing energy have to be equal on average so that the energy budget at the top of the atmosphere balances. About 29 percent of the incoming solar energy arriving at the top of the atmosphere is reflected back to space by clouds, atmospheric particles, or reflective ground surfaces like sea ice and snow. About 23 percent of incoming solar energy is absorbed in the atmosphere by water vapor, dust, and ozone. The remaining 48 percent passes through the atmosphere and is absorbed at the surface. Thus, about 71 percent of the total incoming solar energy is absorbed by the Earth system [3].
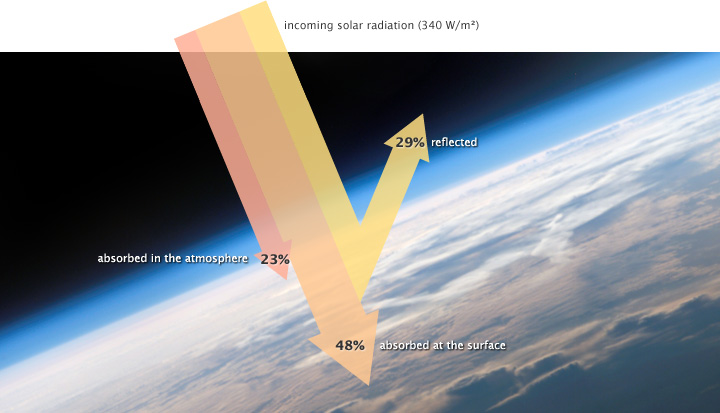
When this energy reaches Earth, the atoms and molecules making up the atmosphere and surface absorb the energy and they increase in temperature. If this material could only absorb energy, then the temperature of the Earth would be like the water level in a sink with no drain where the faucet runs continuously. The sink would eventually overflow. However, the temperature does not infinitely rise because the Earth is not just absorbing sunlight. The Earth’s surface is also radiating thermal energy (heat) back into the atmosphere. If the temperature of the Earth rises, the planet emits an increasing amount of heat to space and this is the primary mechanism that prevents Earth from continually heating [3].
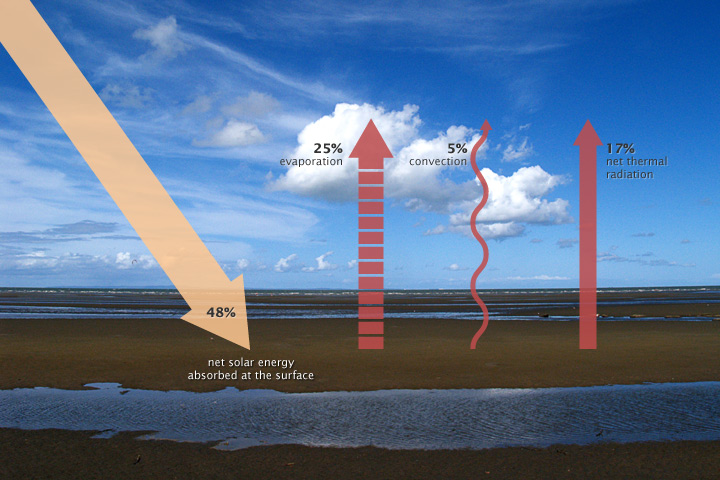
Greenhouse gases act like a giant blanket for Earth. The more greenhouse gases in the atmosphere, then the more outgoing heat will be retained by Earth and the less of this thermal infrared energy (heat) dissipates to space. The greenhouse effect is discussed in more detail in the next section.
Factors that can affect the Earth’s energy budget are not limited to greenhouse gases. Increases in solar irradiance (more solar energy) can increase the energy received by the earth. However, increases associated with this are very small [3; 4; 5]. In addition, less ice and snow covers the land and the Arctic Sea increases the amount of sunlight absorbed by land and water (see animation below). The reflectivity of the Earth’s surface is called albedo. Furthermore, aerosols (dust particles) produced from burning coal, diesel engines, and volcanic eruptions can reflect more incoming solar radiation and actually cool the planet. The effect of anthropogenic aerosols is weak on the climate system but anthropogenic production of greenhouse gases is not weak. Thus, the net effect is warming due to more anthropogenic greenhouse gases associated with fossil fuel combustion [6; 7; 8].
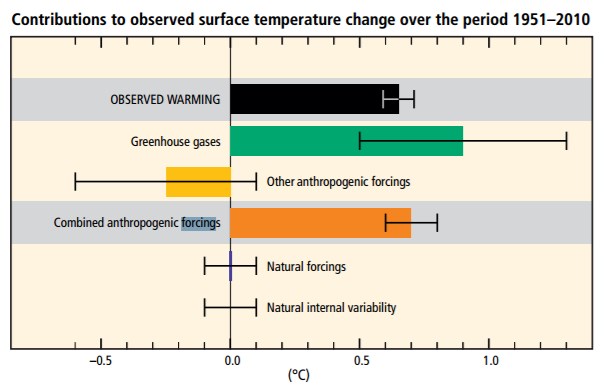
An effect that changes the planet can trigger feedback mechanisms that amplify or suppress the original effect. A positive feedback mechanism is when the output or effect enhances the original stimulus or cause. Thus, it increases the effect later. For example, the loss of sea ice at the North Pole makes that area less reflective (reduced albedo). This allows the surface air and ocean to absorb more energy in an area that was once covered by sea ice [3]. Another example is the melting permafrost. Permafrost is permanently frozen soil located near the high latitudes, mostly in the Northern Hemisphere. As the climate warms, more permafrost thaws and the thick deposits of organic matter are exposed to oxygen and begin to oxidize (or decay). This oxidation process releases carbon dioxide and methane which in turn causes more warming which melts more permafrost, etc.
A negative feedback mechanism occurs when the output or effect reduces the original stimulus or cause [3]. For example, in the short term, more carbon dioxide (CO2) is expected to cause forest canopies to grow and absorb more CO2. An example of the long term is increased carbon dioxide (CO2) in the atmosphere is expected to cause more carbonic acid and chemical weathering, resulting in transport of dissolved bicarbonate and other ions to the oceans which then become stored in sediment.
Composition of Atmosphere
![By Mysid [Public domain], <a data-cke-saved-href="https://commons.wikimedia.org/wiki/File%3AAtmosphere_gas_proportions.svg" href="https://commons.wikimedia.org/wiki/File%3AAtmosphere_gas_proportions.svg" This figure shows the proportion of atmopheric gases at 78% for nitrogen, 21% for oxygen, 1% for argon, and less than 1% for trace components.](http://opengeology.org/textbook/wp-content/uploads/2017/03/15.1_Atmosphere_gas_proportions.png) via Wikimedia Commons" width="200" src="/@api/deki/files/7876/15.1_Atmosphere_gas_proportions.png">
via Wikimedia Commons" width="200" src="/@api/deki/files/7876/15.1_Atmosphere_gas_proportions.png">The composition of the atmosphere is a key component of the regulation of the planet’s temperature. The atmosphere is 78% nitrogen (N2), 21% oxygen (O2), 1% argon (Ar), and less than 1% for all other gases known as trace components. The trace components include carbon dioxide (CO2) water vapor (H2O), neon, helium, and methane. Water vapor is highly variable, mostly based on region, but has been estimated to be about 1% of the atmosphere [9]. The trace gases include several important greenhouse gases, which are the gases responsible for warming and cooling the plant. On a geologic scale, the source of atmospheric CO2 is volcanoes and the sink for CO2 is the weathering process that buries CO2 in sediments. Biological processes both add and subtract CO2 from the atmosphere [10].
Greenhouse gases trap heat in the atmosphere and warm the planet. They have little effect on incoming solar radiation (which is shortwave radiation) but absorb some of the outgoing infrared radiation (longwave radiation) that is emitted from Earth, thus keeping it from being lost to space. More greenhouse gases in the atmosphere absorb more longwave heat and make the planet warmer.
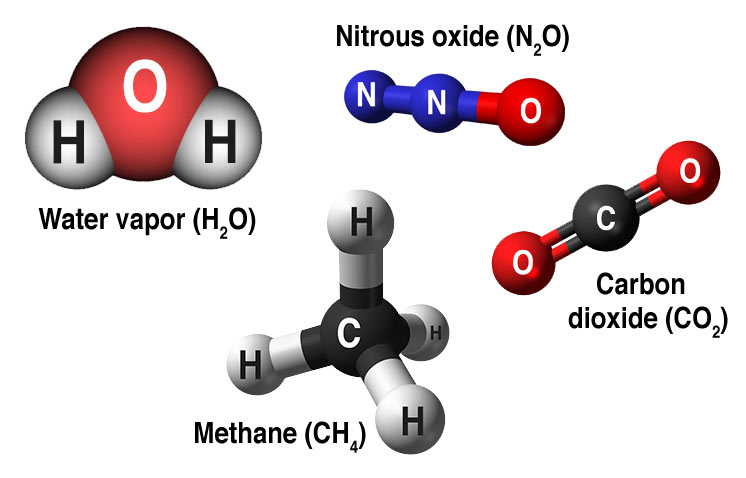
The most common greenhouse gases are water vapor (H2O), carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O). Water vapor is the most abundant greenhouse gas but its abundance in the atmosphere does not change much over time. Carbon dioxide is much less abundant than water vapor, but carbon dioxide is being added to the atmosphere by human activities such as burning fossil fuels, land-use changes, and deforestation. Further, natural processes such as volcanic eruptions add carbon dioxide [3], but at an insignificant rate compared to anthropogenic contributions.
There are two important reasons why carbon dioxide is the most important greenhouse gas. First, carbon dioxide has a long residence time in the atmosphere (meaning that it does not go away for hundreds of years). Second, most of the additional carbon dioxide is “fossil” in origin. That means that it is released by burning fossil fuels. For example, coal is a fossil fuel. Coal is made from plant material created by photosynthesis millions of years ago and stored in the ground. Photosynthesis takes sunlight plus carbon dioxide and creates the carbohydrates of plants. This occurs over millions of years, as a slow process accumulating fossil carbon in rocks and sediments. When we burn coal, we instantaneously release the stored solar energy and the fossil carbon dioxide that took millions of years to accumulate in the first place.
Carbon Cycle
Earth has two important carbon cycles. One is the biological one, wherein living organisms—mostly plants—consume carbon dioxide from the atmosphere to make their tissues through photosynthesis, and then, after they die, that carbon is released back into the atmosphere when they decay over several years or decades [11]. The following is the general equation for photosynthesis.
CO2 + H2O + sunlight → sugar + O2
The second is the geologic carbon cycle. A small portion of this biological-cycle carbon becomes buried in sedimentary rocks during the slow formation of coal, as tiny fragments and molecules in organic-rich shale, and as the shells and other parts of marine organisms in limestone. This then becomes part of the geological carbon cycle, a cycle that actually involves a majority of Earth’s carbon, but one that operates only very slowly [11].
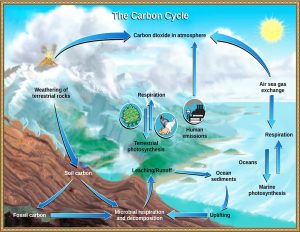
Carbon cycle.
The following is a list of storage reservoirs for the geological carbon cycle.
- Organic matter from plants is stored in peat, coal, and permafrost for thousands to millions of years.
- Weathering of silicate minerals converts atmospheric carbon dioxide to dissolved bicarbonate, which is stored in the oceans for thousands to tens of thousands of years.
- Dissolved carbon is converted by marine organisms to calcite, which is stored in carbonate rocks for tens to hundreds of millions of years.
- Carbon compounds are stored in sediments for tens to hundreds of millions of years; some end up in petroleum deposits.
- Carbon-bearing sediments are transferred by subduction to the mantle, where the carbon may be stored for tens of millions to billions of years.
- During volcanic eruptions, carbon dioxide is released back to the atmosphere, where it is stored for years to decades [11].
During much of Earth’s history, the geological carbon cycle has been balanced, with carbon being released by volcanism at approximately the same rate that it is stored by the other processes. Under these conditions, the climate remains relatively stable. During some times of Earth’s history, that balance has been upset. This can happen during prolonged stretches of greater than average volcanism. One example is the eruption of the Siberian Traps at around 250 million years ago, which appears to have led to strong climate warming over a few million years. A carbon imbalance is also associated with significant mountain-building events. For example, the Himalayan Range has been forming since about 40 Ma and over that time — and still today — the rate of weathering on Earth has been enhanced because those mountains are so high and the range is so extensive. The weathering of these rocks — most importantly the hydrolysis of feldspar — has resulted in the consumption of atmospheric carbon dioxide and transfer of the carbon to the oceans and to ocean-floor carbonate minerals. The steady drop in carbon dioxide levels over the past 40 million years, which contributed to the Pleistocene glaciations, is partly attributable to the formation of the Himalayan Range. Another, non-geological form of carbon-cycle imbalance is happening today on a very rapid time scale. We are in the process of extracting vast volumes of fossil fuels (coal, oil, and gas) that were stored in rocks over the past several hundred million years, and converting these fuels to energy and carbon dioxide. By doing so, we are changing the climate faster than has ever happened in the past [11].
Greenhouse Effect
The greenhouse effect is a natural process by which the atmosphere warms surface temperatures. Without an atmosphere, Earth would have huge fluctuations in temperature between day and night like the moon. Daytime temperatures would be hundreds of degrees Fahrenheit above normal and nighttime temperatures would be hundreds of degrees below normal. The greenhouse effect occurs because of the presence of greenhouse gases in the atmosphere.
The greenhouse effect is named after a similar process that warms a greenhouse or a car on a hot summer day. Sunlight passes through the glass of the greenhouse or car, reaches the interior, and changes into heat. The heat radiates upward and gets trapped by the glass windows. The greenhouse effect for the Earth can be explained in three steps.
Step 1: Solar radiation from the sun is composed of mostly ultraviolet (UV), visible light, and infrared (IR) radiation. Components of solar radiation include parts with a shorter wavelength than visible light, like ultraviolet light, and parts of the spectrum with longer wavelengths, like IR and others. Some of the radiation gets absorbed, scattered, or reflected by the atmospheric gases but about half of the solar radiation eventually reaches the Earth’s surface.
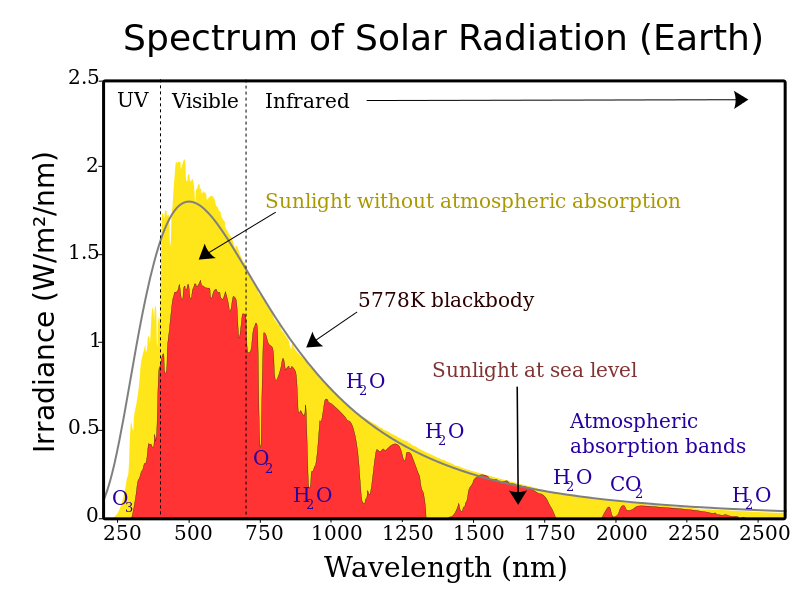 CC BY-SA 3.0], via Wikimedia Commons" width="800" src="/@api/deki/files/7883/15.1_Solar_Spectrum.png">
CC BY-SA 3.0], via Wikimedia Commons" width="800" src="/@api/deki/files/7883/15.1_Solar_Spectrum.png">Step 2: The visible, UV, and IR radiation, that reaches the surface converts to heat energy. Most students have experienced sunlight warming a surface such as a paved surface, a patio, or deck. When this occurs, the warmer surface thus emits more thermal radiation, which is a type of IR radiation. So, there is a conversion from visible, UV, and IR to just thermal IR. This thermal IR is what we experience as heat. If you have ever felt the heat radiating from a fire or a hot stovetop, then you have experienced thermal IR.
Step 3: Thermal IR radiates from the earth’s surface back into the atmosphere. But since it is thermal IR instead of UV, visible, or regular IR, this thermal IR gets trapped by greenhouse gases. In other words, the sun’s energy leaves the Earth at a different wavelength than it enters, so, the sun’s energy is not absorbed in the lower atmosphere when energy is coming in, but rather when the energy is going out. The gases that typically do this blocking on Earth include carbon dioxide, water vapor, methane, and nitrous oxide. More greenhouse gases in the atmosphere result in more thermal IR being trapped. Explore this external link to an interactive animation on the greenhouse effect from the National Academy of Sciences.


