4.4: Partial Melting and Crystallization
- Page ID
- 6863
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Even though all magmas originate from similar mantle rocks, and start out as similar magma, other things, like partial melting and crystallization processes like magmatic differentiation, can change the chemistry of the magma. This explains the wide variety of resulting igneous rocks that are found all over Earth.
Partial Melting
Because the mantle is composed of many different minerals, it does not melt uniformly. As minerals with lower melting points turn into liquid magma, those with higher melting points remain as solid crystals. This is known as partial melting. As magma slowly rises and cools into solid rock, it undergoes physical and chemical changes in a process called magmatic differentiation.
According to Bowen’s Reaction Series (Section 4.2), each mineral has a unique melting and crystallization temperature. Since most rocks are made of many different minerals, when they start to melt, some minerals begin melting sooner than others. This is known as partial melting and creates magma with a different composition than the original mantle material.
The most important example occurs as magma is generated from mantle rocks (as discussed in Section 4.3). The chemistry of mantle rock (peridotite) is ultramafic, low in silicates and high in iron and magnesium. When peridotite begins to melt, the silica-rich portions melt first due to their lower melting point. If this continues, the magma becomes increasingly silica-rich, turning ultramafic mantle into mafic magma, and mafic mantle into intermediate magma. The magma rises to the surface because it is more buoyant than the mantle.

Partial melting also occurs as existing crustal rocks melt in the presence of heat from magmas. In this process, existing rocks melt, allowing the magma formed to be more felsic and less mafic than the pre-existing rock. Early in the Earth’s history when the continents were forming, silica-rich magmas formed and rose to the surface and solidified into granitic continents. In the figure, the old granitic cores of the continents, called shields, are shown in orange.
Crystallization and Magmatic Differentiation
Liquid magma is less dense than the surrounding solid rock, so it rises through the mantle and crust. As magma begins to cool and crystallize, a process known as magmatic differentiation changes the chemistry of the resultant rock towards a more felsic composition. This happens via two main methods: assimilation and fractionation [8].

During assimilation, pieces of country rock with a different, often more felsic, composition are added to the magma. These solid pieces may melt, which changes the composition of the original magma. At times, the solid fragments may remain intact within the cooling magma and only partially melt. The unmelted country rocks within an igneous rock mass are called xenoliths.
Xenoliths are also common in the processes of magma mixing and rejuvenation, two other processes that can contribute to magmatic differentiation. Magma mixing occurs when two different magmas come into contact and mix, though at times, the magmas can remain heterogeneous and create xenoliths, dikes, and other features. Magmatic rejuvenation happens when a cooled and crystallized body of rock is remelted and pieces of the original rock may remain as xenoliths.
Much of the continental lithosphere is felsic (i.e. granitic), and normally more buoyant than the underlying mafic/ultramafic mantle. When mafic magma rises through thick continental crust, it does so slowly, more slowly than when magma rises through oceanic plates. This gives the magma lots of time to react with the surrounding country rock. The mafic magma tends to assimilate felsic rock, becoming more silica-rich as it migrates through the lithosphere and changing into intermediate or felsic magma by the time it reaches the surface. This is why felsic magmas are much more common within continents.
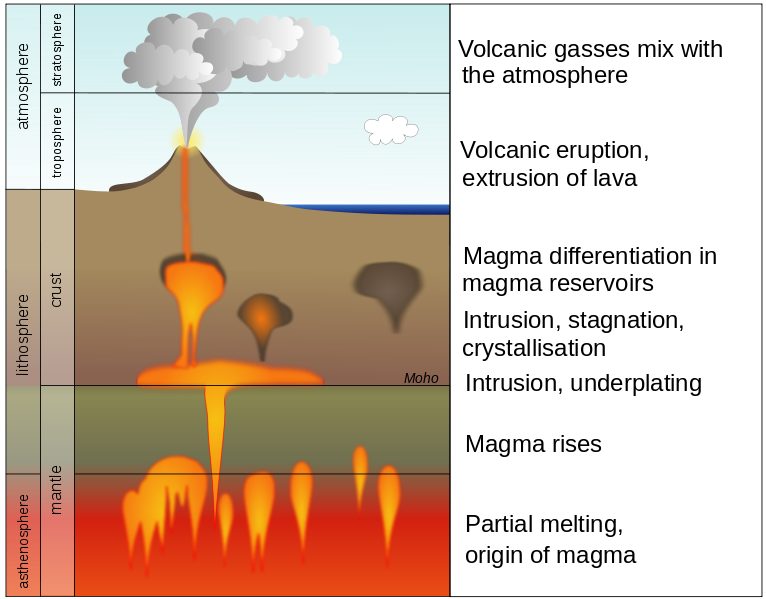
Fractionation or fractional crystallization is another process that increases the magma silica content, making it more felsic [9]. As the temperature drops within a magma diapir rising through the crust, some minerals will crystallize and settle to the bottom of the magma chamber, leaving the remaining melt depleted of those ions. Olivine is a mafic mineral at the top of the Bowen’s Reaction series with a high melting point and a smaller percentage of silica versus other common igneous minerals. When ultramafic magma cools, the olivine crystallizes first and settles to the bottom of the magma chamber (see figure). This means the remaining melt becomes more silica-rich and felsic. As the mafic magma further cools, the next minerals on Bowen’s Reaction Series (plagioclase and pyroxene) crystallize next, removing even more low-silica components from the magma, making it even more felsic. This crystal fractionation can occur in the oceanic lithosphere, but the formation of more differentiated, highly evolved felsic magmas is largely confined to continental regions where the longer time to the surface allows more fractionation to occur.

Figure \(\PageIndex{1}\): Schematic diagram illusrating fractional crystallization. If magma at composition A is ultramafic, as the magma cools it changes composition as different minerals crystallize from the melt and settle to the bottom of the magma chamber. In section 1, olivine crystallizes; section 2: olivine and pyroxene crystallize; section 3: pyroxene and plagioclase crystallize; and section 4: plagioclase crystallizes. The crystals are separated from the melt and the remaining magma (composition B) is more silica-rich. (Source: Woudloper)


