3.1: Atmospheric Composition
- Page ID
- 15171
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Our atmosphere is a dynamic mixture of gases that envelop the Earth. Two gases, nitrogen and oxygen, make up most of the atmosphere by volume. They are indeed important for maintaining life and driving a number of processes near the surface of the Earth. Many of the so called "minor gases" (known here as "variable gases") play an equally important role in the Earth system. These gases include those that have a significant impact on the heat budget and the availability of moisture across the Earth. The atmosphere is not a homogeneous mass of gases, but has a layered structure as defined by vertical temperature changes.
Two broad regions can be identified using air composition as a means to subdivide the atmosphere. The heterosphere is the outer most sphere where gases are distributed in distinct layers by gravity according to their atomic weight. Extending from an altitude of 80 km (50 mi), the lightest elements (hydrogen and helium) are found at the outer margins of the atmosphere. The heavier elements (nitrogen and oxygen) are found at the base of the layer.
The homosphere lies between the Earth's surface and the heterosphere. Gases are nearly uniformly mixed through this layer even though density decreases with height above the surface. The only exceptions is the "ozone layer" from 19 to 50 km (12 to 31 mi) and near surface variations in water vapor, carbon dioxide and air pollutants.
Constant Gases
Nitrogen, oxygen and argon are called the "constant gases" because their concentration has remained virtually the same for much of recent earth history. Nitrogen (78%)is a relatively inert gas produced primarily by volcanic activity. It is an important component of protein in meat, milk, eggs and the tissues of plants, especially grains and members of the pea family. It cannot be ingested directly by organisms but made available to plants, and then to animals, by compounds in the soil. Most atmospheric nitrogen enters the soil by nitrogen-fixing microorganisms.
Oxygen (21%) is important for plant and animal respiratory processes. It is also important to chemical reactions (oxidation) that breakdown rock materials (chemical weathering). Without oxygen, things cannot burn either. Free oxygen in the atmosphere is a product of plant photosynthesis. Plants take up carbon dioxide and in the process of photosynthesis release oxygen.
Argon (0.93%) is a colorless, odorless relatively inert gas, the reason it use to electric light bulbs, fluorescent tubes. It is used to form inert atmosphere for arc welding, and growing semiconductor crystals.
Variable Gases
The so called "variable gases" are those present in small and variable amounts. These include carbon dioxide, methane, ozone, water vapor, and particulates among others. Even though they represent a tiny portion of the atmosphere as a whole, they exert a great control over our environment.
Carbon dioxide
Carbon dioxide(CO2) makes up only 0.036% of the atmosphere by volume. Carbon dioxide is essential to photosynthetic processes of plants. Huge quantities of carbon are stored in plant tissue, deposits of coal, peat, oil, and gas. Carbon dioxide is taken in by plants and during photosynthesis is combined with water and energy to form oxygen and carbohydrates. The stored carbohydrates are used to fuel plant respiration and growth. Carbon is also stored in limestone rocks that have formed by the compaction of carbonate-rich shells of ocean life. Because vegetation takes in so much carbon dioxide, we often refer to plants as a "sink" for it.
Carbon dioxide in the atmosphere varies throughout the year, decreasing slightly during the summer as plants leaf out, and then increases during the winter as plants go dormant and photosynthesis decreases. The zigzag pattern of carbon dioxide measurements taken at Mauna Loa, Hawaii in Figure \(\PageIndex{1}\) below illustrates this seasonality.
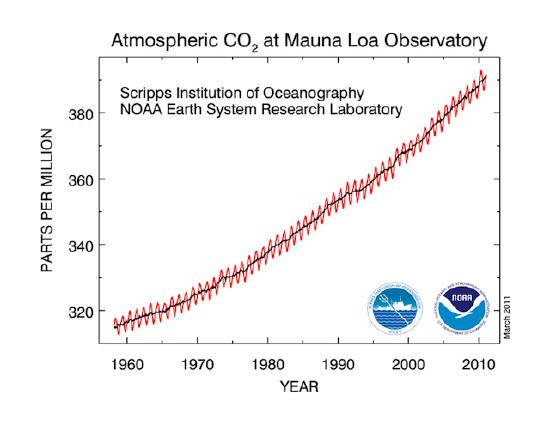
The seasonal changes in the geography of uptake and release of carbon dioxide during 2004 is shown in Figure \(\PageIndex{2}\) by NOAA's CarbonTracker. The black and white dots are locations where CO2 data is collected. A computer model based on this data and a knowledge of surface sources and sinks generates the patterns for the entire globe. Again, the large season to season variation is due to plant life. Blue colors over the northern hemisphere during July in the midlatitudes result from forests and crops soaking up large quantities of CO2. Intense red areas of CO2 release during July, August, and September in the southern hemisphere is largely due to biomass burning. Some burning is natural, such as the dry savanna grasses ignited by lightning strikes. Most is due to people burning fields in preparation for planting, or burning of forests for new agricultural land.
Video: NOAA's Carbon Tracker (Courtesy NOAA Earth System Research Laboratory)
Methane
Methane (CH4) is a greenhouse gas contributing to about 18% of global warming and has been on the rise over the last several decades. Though methane makes up far less of the atmosphere (.0002%) than carbon dioxide, it is 20 times more potent than CO2 as a greenhouse gas. Methane is a product of the decomposition of organic matter, with major natural sources being that which occurs from wetlands, termites, the oceans, and hydrates.
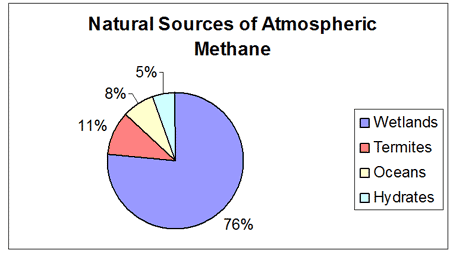
A major source of methane is from termites. Termites eat wood and produce methane as a result of the breakdown of cellulose in their digestive tracts. They are thought to be responsible for 11% of the methane in the atmosphere (some estimates are as high as 20% - 40%). The clearing of the rain forests greatly impacts termite populations and in turn the methane content of the atmosphere. When a patch of rain forest is cleared, termite populations explode due to the ample food source that is left behind.

Human activities have contributed to the rise of methane in our atmosphere. Landfills, rice paddy agriculture, natural gas systems, and livestock production appear to be significant contributors of anthropogenic sources of methane.
Ozone
Ozone (O3) is both beneficial and harmful to life on Earth. Much of the ozone in the atmosphere is found in the stratosphere. Here, ozone absorbs UV light from the Sun preventing it from reaching the surface. Without this blanket, humans would be exposed to serious sun burn and potential risk of skin cancer. Ozone is also found in the lowest layer of the atmosphere, the troposphere. Here ozone can act as an eye and respiratory irritant. Ozone also causes cellular damage inside the leaves of plants causing brown splotches, impairing carbon dioxide uptake and disrupting the photosynthetic apparatus. Such damage can cause economic losses through reduced crop yields. It also damages the carbon "sink" role of vegetation leaving more carbon dioxide in the atmosphere to enhance the greenhouse effect and potential global warming.
Human-produced compounds such as chlorofluorocarbons and halides containing chlorine and bromine destroy ozone, and have disrupted the fragile stratospheric ozone layer over Antarctica and the Arctic. Ozone depletion over Antarctica occurs during the spring when sunlight returns to the South Pole and the temperatures are still very cold.
Video: Exploring Ozone (Courtesy NASAexplorer)
Water Vapor
Water vapor is an extremely important gas found in the atmosphere. It can vary from 4% in the steamy tropics to nearly nonexistent in the cold dry regions of the Antarctic. Water vapor is a good absorber of Earth's outgoing radiation and thus is considered a greenhouse gas. When water vapor is converted to a liquid during condensation, clouds are formed. Clouds are good absorbers of radiation given off by the Earth's surface. The absorption of this energy raises the temperature of the air. But clouds are generally light-colored and hence reflect incoming solar radiation off their tops. The reflected light is sent back to space, never reaching the ground to warm the Earth. Thus clouds can have either a warming or a cooling effect on air temperature. It has been thought that these effects balance one another out but National Public Radio's All Things Considered report suggests that this might not be true, forcing climatologists to rethink the issue.
Particulates and Aerosols
Atmospheric particulates and aerosols are very small particles of solid or liquid suspended in the air. Particulates and aerosols play several important roles in atmospheric processes. Particulate matter includes dust, dirt, soot, smoke, and tiny particles of pollutants. Major natural sources of particulates are volcanoes, fires, wind-blown soil and sand, sea salt, and pollen. Human sources such as factories, power plants, trash incinerators, motor vehicles, and construction activity also contribute particulates to the atmosphere.
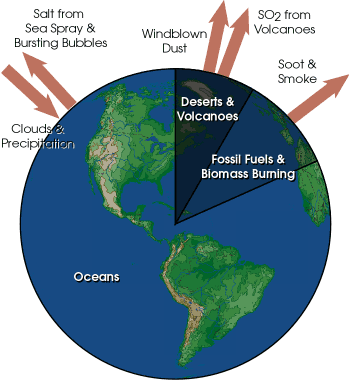
Particulates are very effective at altering the energy and moisture balances of the Earth system. Particulates diffuse sunlight reducing the amount and intensity of solar radiation reaching the Earth's surface. The most spectacular sunrises and sunsets are a result of light being refracted from particulates in the atmosphere. Particulates will also reflect sunlight back out to space, never letting it reach the surface. Decreasing significant amounts of incoming solar radiation can cause global temperatures to decrease. The eruption of Mt. Pinatubo in 1991 caused a .5o C decrease in global temperatures. However, particulates can absorb longwave radiation emitted by the Earth, causing the atmosphere to warm as well. Particulates serve as condensation nuclei for water. In order for water to change from a gas to a liquid, a nucleus upon which water vapor can attach itself is nearly always required. Without particulates, little water would condense to form clouds and precipitation.

NASA scientists using satellite data and computer models found black soot from incomplete combustion may be contributing to changes in sea ice, snow and atmospheric temperatures near the North Pole. They found the timing and location of rising temperatures and loss of sea ice during the end of the 20th century is consistent with a significant rise in human produced aerosols. Their models suggest that one third of the soot comes from South Asia, one third from biomass burning, and the rest from Russia, Europe, and North America. Soot deposited on snow and sea ice decreases the surface reflectivity causing more sunshine to be absorbed. Airborne soot warms the Arctic atmosphere and affects weather patterns and clouds.
Video: Climate Change and Aerosols
Dr. Jim Haywood, Aerosol Research scientist at the Met Office talks about climate change and aerosols.
Assess you understanding of the preceding material by "Looking Back: Atmospheric Composition" or skip and continue reading
Assess your understanding of concepts related to this chapter section by answering the questions below before proceeding with this chapter. Click the "Answer" to reveal the correct answer.
Define homosphere and heterosphere
- Answer
-
Homosphere: Sphere that lies between the surface and heterosphere where gases are uniformly mixed. Heterosphere: Outermost "sphere" of the atmosphere where gases are distributed into distinct layers.
List the "permanent (constant)" gases.
- Answer
-
nitrogen, oxygen, argon
Which greenhouse gas is more effective at heating the atmosphere, carbon dioxide or methane?
- Answer
-
Methane
Which gas filters UV light in the stratosphere, carbon dioxide, ozone, or methane?
- Answer
-
Ozone.
Soot increases or decreases reflectivity resulting in melting when deposited on snow?
- Answer
-
Decreases


