2.6: The Mineral Particles of the Earth's Surface Materials
- Page ID
- 14250
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)2.6.1
It seems a good idea, at this point, to describe the most common kinds of mineral particles in the ground under your feet—the materials that in the sections to follow are called regolith, sediment, and soil (regolith being the most inclusive term, as you will see). I think it’s accurate to say that most regolith consists mostly of mineral particles, coarse and fine. A wide variety of kinds of minerals can be found in typical regolith, but only a very few are of any abundance.
2.6.2
In terms of relatively coarse mineral particles, at the top of the list is quartz. Quartz is common in most of the source rocks from which surface materials are derived, and it’s largely immune to chemical weathering. This is a first-order fact: most of the mineral grains in most of the regolith you’re ever going to see (with the notable exceptions of fine muds) consist of quartz. Quartz grains are mostly equant (meaning approximately equidimensional) in “overall” shape, and usually in between subangular (that is, a bit more rounded than a classically jaggedy angular particle) and well rounded in “local” shape. The degree of roundness depends partly on the shape of the particle upon being freed from the parent rock by weathering and partly on the extent of mechanical wear the quartz particle has experienced, and perhaps also on some dissolution if the quartz particle is exposed to acidic environments (because the solubility of quartz in water, although extremely low in pure water, increases markedly with increasing acidity of the water).
2.6.3
Potassium feldspar is also important as coarse particles in regolith, depending upon the composition of the parent rock. Potassium feldspar is more susceptible to weathering than is quartz, but commonly some or even much of it escapes weathering before it is deposited as sediment and passes out of the reach of near-surface processes. Survival of potassium feldspar to be seen in regolith is especially common in situations where weathering is curtailed, either by a cold and rigorous climate or by rapid stripping of the weathered material from steep slopes. Commonly, potassium feldspar in regolith is partly or mostly weathered to kaolinite, and under magnification has a “punky” or “chalky” appearance.
2.6.4
Rock fragments are also common in regolith. This is especially the case for sediments. Imagine a raging river eating its way into fractured or partly weathered bedrock: it can entrain fragments of even fresh rock, large and small, and transport them to sites of eventual deposition. Glaciers can do the same thing even more effectively. Identifying large rock fragments is easy; also important, however, are sand-size fragments of various common fine-grained rocks. It takes careful microscopic work to identify such fragments.
2.6.5
Muscovite (the most common form of mica, but not the only one) as coarse particles is common in regolith as well. Micas are sheet-silicate minerals. Sheet silicates are those in which the silica tetrahedra are polymerized by sharing of three of the four oxygens to form a sheet structure, with hexagonal symmetry, and with all of the unshared oxygens pointing in the same direction. Figure 2-10 is a drawing of the sheet structure, looking down on the side with the unshared oxygens. There’s more to the mica structure than that, however: the basic sheet unit consists of two such silica sheets, each with their unshared oxygens pointing inward, with aluminum ions in between the two silica sheets. (If you want more detail on mica structure, see the clay mineral “advanced topic” below.)
2.6.6
The mica crystal is very weak in the direction parallel to the sheets of the structure, so in regolith it’s usually in the form of plates or flakes. When it’s coarse, so it can be seen with the unaided eye or with a hand lens, it is easy to identify. In sediments it’s usually noticeably coarser than the equant particles like quartz, because its platy shape makes it easier for flowing fluid to transport it
2.6.7
In terms of relatively fine mineral particles in regolith, at the top of the list is the group of clay minerals. These are a group of minerals rather than a single mineral. Clay minerals are mainly sheet-silicate minerals. Some, called illite or smectite, have the same structure as muscovite, and nearly the same composition. Owing to various substitutions of some ions for others, though, the range of compositions of illite and smectite is wide. In fact, sedimentologists who have to deal with clay minerals often just refer to illite—smectite (or I–S for short). Kaolinite and chlorite, with yet different sheet structures, are other common clay minerals. For more detail on the nature of clay-mineral structures, see the following optional “advanced topic”. The proportions of the various clay-mineral types in a given sample of regolith vary depending upon two major factors: source-rock composition, and weathering environment
2.6.8
An important thing for you to know about clay minerals is that clay- mineral particles are all very small, mostly less than a few micrometers. They range in size down to very small fractions of a micrometer, into what is called the colloidal size range. There will be more on colloids in the later section on soils, where they are especially significant.
Advanced Topic: Clay Minerals
I. Introduction
The word “clay” is used, somewhat ambiguously and confusingly, in three different but largely overlapping senses:
(1) As a mineral term, for a group of minerals, mostly but not entirely sheet silicates, that are produced from weathering of aluminosilicate minerals (mainly feldspars). They are almost always very fine, from the sub-micrometer range up to several micrometers, and they are mostly platy in shape, although some are curvy and curled. The principal kinds are kaolinite, smectites, illites, and chlorites. (I’ve used the plural for the last three because there’s a very wide compositional range in each of them.)
(2) As a size term. According to the official grade scale for sediment sizes (see the later section on sediment), all particles finer than 1/256 of a millimeter, or about 4 micrometers, are termed clay.
(3) As a material term (“clay material”, or just “clay”), which has a sticky and tenacious consistency. It’s the stuff that potters use.
Most clay-mineral particles are of clay size; most clay-size particles are clay-mineral particles; and most clay material consists of clay-mineral particles of clay size!
As you can imagine, clay minerals are not easy to study: even in the coarser size range it’s difficult to see them microscopically. Standard techniques for study include electron microscopy, which allows you to see the particle shapes beautifully, and x-ray diffraction, which allows you to identify their mineralogy, after a fashion. More sophisticated instrumental techniques have been developed more recently. Clay mineralogy is worth a whole course in itself. What I'll do here is just give you a cursory account of the most important clay minerals and their occurrence.
II. Structure
Two structural units are involved in clay-mineral structures:
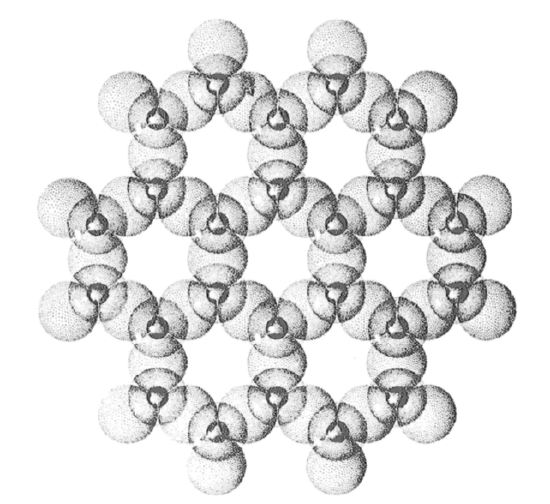
octahedral sheets: two planes of closely packed oxygens and hydroxyls (OH-) with a plane of Al, Fe, and/or Mg between. The Al, Fe, and/or Mg are in octahedral coordination with the O and OH; that is, each of the former is equidistant from six of the latter, which are arranged as an octahedron around the former. Figure 2-11 shows a rough sketch of the arrangement. Figure 2-12 is a more detailed view of the geometry, perpendicular to the sheet. The oxygen-to-oxygen distance is 2.94 Å, and the thickness of the sheet is 5.05 Å. If Al is in the octahedral positions, only two-thirds of the positions are filled; if Mg and/or Fe are in the octahedral positions, all of the positions are filled.
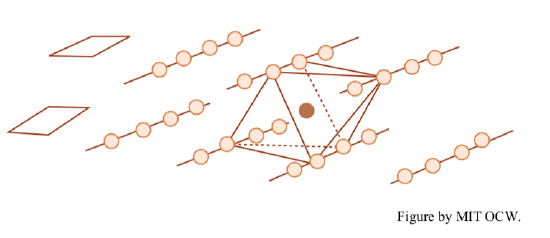
tetrahedral sheets, in which silica (SiO4) tetrahedra are polymerized by sharing of three of the four oxygens to form a sheet structure with hexagonal symmetry. Figure 2-13 shows two diagrammatic representations. The oxygen-to-oxygen distance is 2.55 Å, and the thickness of the sheet is 4.65 Å.
These two layers, or sheetlike arrangements, fit together, one on top of the other, in such a way that the vertices of the tetrahedra in the tetrahedral sheet point toward the octahedral sheet. The unshared oxygens of the tetrahedral sheet and the oxygens of the octahedral sheet are the same; they are shared for double duty. The hydroxyls that form part of the octahedral arrangement fit into the hexagonal holes of the tetrahedral sheet.
The dimensions of the two sheets are almost but not quite right for this shared arrangement. There has to be some strain in the lattices to make them go together to share oxygens. This seems to be accommodated by alternate cocking of the tetrahedra, to contract the tetrahedral sheet a little, and stretching and thinning of the octahedral sheet to make it a little bigger. I'll schematize this two-layer structure as shown in Figure 2-14.

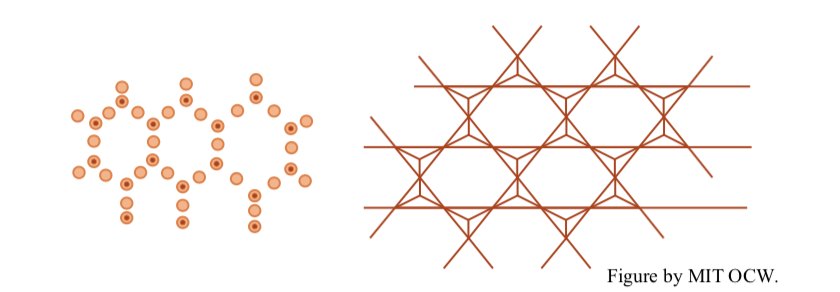
It's also possible to construct a three-layer arrangement in which a tetrahedral layer is sandwiched between two tetrahedral layers. Figure 2-15 shows the highly schematic way I’ll represent this three-layer structure.
III. The Major Clay Minerals
Kaolinite:
Kaolinite (Figure 2-16) is the main two-layer mineral. It's fairly simple chemically: Al4(Si4O10)(OH)8, with no isomorphous substitutions in either the tetrahedral sheet or the octahedral sheet; it’s the purest of the clay minerals. Only two-thirds of the octahedral positions are filled, by aluminum; minerals like that are called dioctahedral. There’s no net charge on the tetrahedral–octahedral layers; the layers are held together only by a kind of weak bonds called van der Waals bonds. The repeat distance normal to the layers is 7.2 Å, so this group of minerals is sometimes called seven-Ångstrom clays. The crystals can get relatively large, because there’s very little stress in the structure; kaolinite is the coarsest of the clay minerals. Kaolinite is formed mainly by weathering of feldspars; production of kaolinite is favored by acidic and warm environments.
Illite:
Illite (Figure 2-17) is a hydrous muscovite-like clay mineral. It has the same three-layer structure as muscovite, but less than the one-in-four replacement of Si by Al that’s characteristic of muscovite (the figure is between one-in-five and one-in-six). So there isn’t the same charge deficiency as in muscovite, and not as many K+ ions in the interlayer positions. H3O+ ions are also present in the interlayer positions. Also, substitutions in the octahedral layer are greater and more random; there’s lots of variability. Illite is never found well crystallized, only as clay-size particles. The particle size is typically less than one micrometer. This is because there’s considerable stress in the layers. Illite is closely similar in structure to the common muscovite you see in sedimentary, metamorphic, and igneous rocks; it’s just much finer-grained. Illite is formed mainly by weathering of potassium feldspar in temperate weathering environments. With increasing intensity of weathering, kaolinite is formed instead.
Chlorite:
Chlorite (Figure 2-18) is a kind of mixed-layer clay mineral. Its structure is an alternation of trioctahedral TOT layers, with Mg2+ and Fe2+ in the octahedral positions, and layers with the composition (Mg2+, Fe2+)3(OH)6, giving a repeat spacing of about 14 Å. There’s a great range and diversity of ionic substitutions in each of the three ion sites, as well as differences in the geometry of stacking of the sheets Chlorite is produced in abundance only in relatively mild weathering environments, where it survives from the source rocks, although in the form of finer particles. So it’s characteristically a high-latitude product.
2.6.9
Various oxides of iron and aluminum are especially important in the highly weathered soils of warm and humid regions, where (as you saw in the section on weathering) silicate minerals are broken down further to oxides. The main aluminum oxide is gibbsite. The formula for gibbsite, Al(OH)3, shows that it is a hydroxide mineral. Gibbsite is formed by further weathering of silicate clay minerals, in which all of the silica is stripped out of the clay minerals; see Reaction 9 in the section on chemical weathering, above. The main iron oxide mineral in soils is goethite, FeOOH. It’s goethite that imparts the characteristic yellow-orange color to weathered iron-bearing rocks and regolith. Recall from the section of chemical weathering that ferrous iron is freed from ferrous-iron-bearing silicate minerals and then oxidized to minerals like goethite. These aluminum and iron oxides (there are others, as well) in regolith are in the form of extremely small particles, in the colloidal size range. (See the later section on soils for more on colloids.)